Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial
- PMID: 35057489
- PMCID: PMC8780623
- DOI: 10.3390/nu14020309
Prebiotic Galacto-Oligosaccharides Impact Stool Frequency and Fecal Microbiota in Self-Reported Constipated Adults: A Randomized Clinical Trial
Abstract
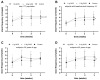
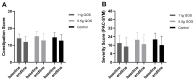
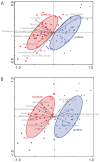
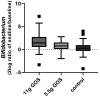
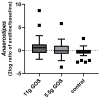
Constipation is a major issue for 10-20% of the global population. In a double-blind randomized placebo-controlled clinical trial, we aimed to determine a dose-response effect of galacto-oligosaccharides (GOS) on stool characteristics and fecal microbiota in 132 adults with self-reported constipation according to Rome IV criteria (including less than three bowel movements per week). Subjects (94% females, aged: 18-59 years) received either 11 g or 5.5 g of BiotisTM GOS, or a control product, once daily for three weeks. Validated questionnaires were conducted weekly to study primarily stool frequency and secondary stool consistency. At base- and endline, stool samples were taken to study fecal microbiota. A trend towards an increased stool frequency was observed after the intervention with 11 g of GOS compared to control. While during screening everybody was considered constipated, not all subjects (n = 78) had less than three bowel movements per week at baseline. In total, 11 g of GOS increased stool frequency compared to control in subjects with a low stool frequency at baseline (≤3 bowel movements per week) and in self-reported constipated adults 35 years of age or older. A clear dose-response of GOS was seen on fecal Bifidobacterium, and 11 g of GOS significantly increased Anaerostipes hadrus. In conclusion, GOS seems to be a solution to benefit adults with a low stool frequency and middle-aged adults with self-reported constipation.
Keywords: constipation; galacto-oligosaccharides; microbiota; stool characteristics; stool consistency; stool frequency.
Conflict of interest statement
M.H.S., J.H.J.H., D.H., A.N. and R.B. are employees of FrieslandCampina. The authors declare no conflict of interest.
Figures
References
-
- Serra J., Pohl D., Azpiroz F., Chiarioni G., Ducrotté P., Gourcerol G., Hungin A.P.S., Layer P., Mendive J.-M., Pfeifer J., et al. European Society of Neurogastroenterology and Motility Guidelines on Functional Constipation in Adults. Neurogastroenterol. Motil. 2020;32:e13762. doi: 10.1111/nmo.13762. - DOI - PubMed
-
- Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudo S., et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

