Coal dust exposure triggers heterogeneity of transcriptional profiles in mouse pneumoconiosis and Vitamin D remedies
- PMID: 35057792
- PMCID: PMC8772169
- DOI: 10.1186/s12989-022-00449-y
Coal dust exposure triggers heterogeneity of transcriptional profiles in mouse pneumoconiosis and Vitamin D remedies
Abstract
Background: Coal dust particles (CDP), an inevitable by-product of coal mining for the environment, mainly causes coal workers' pneumoconiosis (CWP). Long-term exposure to coal dust leads to a complex alternation of biological processes during regeneration and repair in the healing lung. However, the cellular and complete molecular changes associated with pulmonary homeostasis caused by respiratory coal dust particles remain unclear.
Methods: This study mainly investigated the pulmonary toxicity of respirable-sized CDP in mice using unbiased single-cell RNA sequencing. CDP (< 5 μm) collected from the coal mine was analyzed by Scanning Electron Microscope (SEM) and Mass Spectrometer. In addition, western blotting, Elisa, QPCR was used to detect gene expression at mRNA or protein levels. Pathological analysis including HE staining, Masson staining, immunohistochemistry, and immunofluorescence staining were performed to characterize the structure and functional alternation in the pneumoconiosis mouse and verify the reliability of single-cell sequencing results.
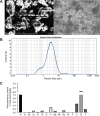
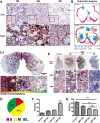
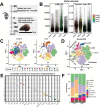
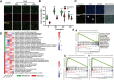
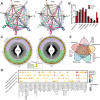
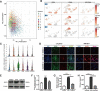
Results: SEM image and Mass Spectrometer analysis showed that coal dust particles generated during coal mine production have been crushed and screened with a diameter of less than 5 µm and contained less than 10% silica. Alveolar structure and pulmonary microenvironment were destroyed, inflammatory and death (apoptosis, autophagy, and necrosis) pathways were activated, leading to pneumoconiosis in post 9 months coal dust stimulation. A distinct abnormally increased alveolar type 2 epithelial cell (AT2) were classified with a highly active state but reduced the antimicrobial-related protein expression of LYZ and Chia1 after CDP exposure. Beclin1, LC3B, LAMP2, TGF-ß, and MLPH were up-regulated induced by CDP, promoting autophagy and pulmonary fibrosis. A new subset of macrophages with M2-type polarization double expressed MLPH + /CD206 + was found in mice having pneumoconiosis but markedly decreased after the Vitamin D treatment. Activated MLPH + /CD206 + M2 macrophages secreted TGF-β1 and are sensitive to Vitamin D treatment.
Conclusions: This is the first study to reconstruct the pathologic progression and transcriptome pattern of coal pneumoconiosis in mice. Coal dust had obvious toxic effects on lung epithelial cells and macrophages and eventually induced pulmonary fibrosis. CDP-induced M2-type macrophages could be inhibited by VD, which may be related to the alleviation of the pulmonary fibrosis process.
Keywords: Alveolar regeneration; Coal dust pulmonary disease; Epithelial cells; Macrophage subset phenotype activation; Pulmonary toxicity; Single-cell RNA sequencing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Lancet. Improving occupational health in China. Lancet. 2019;394 10197:443; doi: 10.1016/S0140-6736(19)31799-4. - PubMed
-
- Zosky GR, Hoy RF, Silverstone EJ, Brims FJ, Miles S, Johnson AR, et al. Coal workers' pneumoconiosis: an Australian perspective. Med J Aust. 2016;204 11:414–8. - PubMed
-
- Perret JL, Plush B, Lachapelle P, Hinks TS, Walter C, Clarke P, et al. Coal mine dust lung disease in the modern era. Respirology. 2017;22 4:662–70; doi: 10.1111/resp.13034. - PubMed
-
- Leon-Mejia G, Silva LF, Civeira MS, Oliveira ML, Machado M, Villela IV, et al. Cytotoxicity and genotoxicity induced by coal and coal fly ash particles samples in V79 cells. Environ Sci Pollut Res Int. 2016;23 23:24019–31; doi: 10.1007/s11356-016-7623-z. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

