Histone acetyltransferase NAA40 modulates acetyl-CoA levels and lipid synthesis
- PMID: 35057804
- PMCID: PMC8781613
- DOI: 10.1186/s12915-021-01225-8
Histone acetyltransferase NAA40 modulates acetyl-CoA levels and lipid synthesis
Abstract
Background: Epigenetic regulation relies on the activity of enzymes that use sentinel metabolites as cofactors to modify DNA or histone proteins. Thus, fluctuations in cellular metabolite levels have been reported to affect chromatin modifications. However, whether epigenetic modifiers also affect the levels of these metabolites and thereby impinge on downstream metabolic pathways remains largely unknown. Here, we tested this notion by investigating the function of N-alpha-acetyltransferase 40 (NAA40), the enzyme responsible for N-terminal acetylation of histones H2A and H4, which has been previously implicated with metabolic-associated conditions such as age-dependent hepatic steatosis and calorie-restriction-mediated longevity.
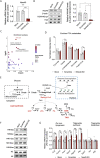
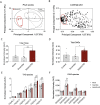
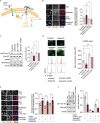
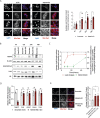
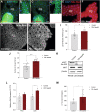
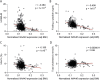
Results: Using metabolomic and lipidomic approaches, we found that depletion of NAA40 in murine hepatocytes leads to significant increase in intracellular acetyl-CoA levels, which associates with enhanced lipid synthesis demonstrated by upregulation in de novo lipogenesis genes as well as increased levels of diglycerides and triglycerides. Consistently, the increase in these lipid species coincide with the accumulation of cytoplasmic lipid droplets and impaired insulin signalling indicated by decreased glucose uptake. However, the effect of NAA40 on lipid droplet formation is independent of insulin. In addition, the induction in lipid synthesis is replicated in vivo in the Drosophila melanogaster larval fat body. Finally, supporting our results, we find a strong association of NAA40 expression with insulin sensitivity in obese patients.
Conclusions: Overall, our findings demonstrate that NAA40 affects the levels of cellular acetyl-CoA, thereby impacting lipid synthesis and insulin signalling. This study reveals a novel path through which histone-modifying enzymes influence cellular metabolism with potential implications in metabolic disorders.
Keywords: Drosophila melanogaster; Epigenetics; Fat body; Histone acetyltransferases; Lipid metabolism; Metabolic disorders; NAA40; acetyl-CoA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

