The use of exhaled air analysis in discriminating interstitial lung diseases: a pilot study
- PMID: 35057817
- PMCID: PMC8772159
- DOI: 10.1186/s12931-021-01923-5
The use of exhaled air analysis in discriminating interstitial lung diseases: a pilot study
Abstract
Background: Fibrotic Interstitial lung diseases (ILD) are a heterogeneous group of chronic lung diseases characterized by diverse degrees of lung inflammation and remodeling. They include idiopathic ILD such as idiopathic pulmonary fibrosis (IPF), and ILD secondary to chronic inflammatory diseases such as connective tissue disease (CTD). Precise differential diagnosis of ILD is critical since anti-inflammatory and immunosuppressive drugs, which are beneficial in inflammatory ILD, are detrimental in IPF. However, differential diagnosis of ILD is still difficult and often requires an invasive lung biopsy. The primary aim of this study is to identify volatile organic compounds (VOCs) patterns in exhaled air to non-invasively discriminate IPF and CTD-ILD. As secondary aim, the association between the IPF and CTD-ILD discriminating VOC patterns and functional impairment is investigated.
Methods: Fifty-three IPF patients, 53 CTD-ILD patients and 51 controls donated exhaled air, which was analyzed for its VOC content using gas chromatograph- time of flight- mass spectrometry.
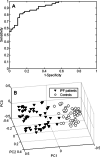
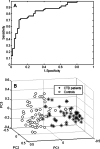
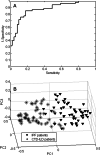
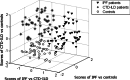
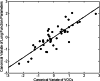
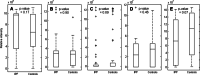
Results: By applying multivariate analysis, a discriminative profile of 34 VOCs was observed to discriminate between IPF patients and healthy controls whereas 11 VOCs were able to distinguish between CTD-ILD patients and healthy controls. The separation between IPF and CTD-ILD could be made using 16 discriminating VOCs, that also displayed a significant correlation with total lung capacity and the 6 min' walk distance.
Conclusions: This study reports for the first time that specific VOC profiles can be found to differentiate IPF and CTD-ILD from both healthy controls and each other. Moreover, an ILD-specific VOC profile was strongly correlated with functional parameters. Future research applying larger cohorts of patients suffering from a larger variety of ILDs should confirm the potential use of breathomics to facilitate fast, non-invasive and proper differential diagnosis of specific ILDs in the future as first step towards personalized medicine for these complex diseases.
Keywords: Connective tissue disease associated-ILD (CTD-ILD); Diagnostic profiles; Gas chromatography–time of flight–mass spectrometry (GC-tof–MS); Idiopathic pulmonary fibrosis (IPF); Volatile organic compound (VOC).
© 2022. The Author(s).
Conflict of interest statement
The authors state that they have no competing interests.
Figures

References
-
- American Thoracic S, European Respiratory S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. - PubMed
-
- Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, Sanyal S, Brillet PY, Brauner M, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J. 2017. 10.1183/13993003.02419-2016. - PubMed
-
- Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146(2):422–436. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

