High-throughput cell-based assays for identifying antagonists of multiple smoking-associated human nicotinic acetylcholine receptor subtypes
- PMID: 35058178
- PMCID: PMC8816891
- DOI: 10.1016/j.slasd.2021.10.001
High-throughput cell-based assays for identifying antagonists of multiple smoking-associated human nicotinic acetylcholine receptor subtypes
Abstract
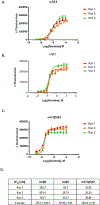
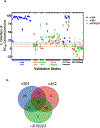
There is substantial evidence that in addition to nicotine, other compounds found in tobacco smoke significantly influence smoking behavior. Further, recent years have seen an explosion in the availability of non-combusted products that deliver nicotine, such as e-cigarettes and "home-brew" vaping devices that are essentially unregulated. There are many thousands of compounds in tobacco smoke alone, and new products are constantly introducing new compounds. Uncovering which of these compounds are active, across multiple smoking-relevant subtypes of the nicotinic acetylcholine receptor (nAChR) that influence tobacco/nicotine addiction, requires a high-throughput screening (HTS) approach. Accordingly, we developed a panel of HTS-friendly cell-based assays, all performed in the same cellular background and using the same membrane potential dye readout, to measure the function of the α3β4-, α4β2-, and α6β2-nAChR subtypes. These subtypes have each been prominently and consistently associated with human smoking behavior. We validated our assays by performing pilot screening of an expanded set of the Prestwick FDA-approved drug library. The screens displayed excellent performance parameters, and moderate hit rates (mean of 1.2% across all three assays) were achieved when identifying antagonists (chosen since effects of endogenous antagonists on consumption of nicotine/tobacco products are under-studied). Validation rates using an orthogonal assay (86Rb+ efflux) averaged 73% across the three assays. The resulting panel of assays represents a valuable new platform with which to screen and identify nAChR subtype-selective compounds. This provides a resource for identifying smoking-related compounds in both combusted and non-combusted tobacco products, with potential relevance in the search for additional smoking-cessation therapies.
Keywords: Cell-based screening; Membrane potential assays; Nicotinic acetylcholine receptor.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Author Disclosure Statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration.J Neurochem. 2014 Jul;130(2):185-98. doi: 10.1111/jnc.12721. Epub 2014 Apr 19. J Neurochem. 2014. PMID: 24661093 Free PMC article.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
-
Electrophysiology-Based Assays to Detect Subtype-Selective Modulation of Human Nicotinic Acetylcholine Receptors.Assay Drug Dev Technol. 2016 Aug;14(6):333-44. doi: 10.1089/adt.2015.688. Assay Drug Dev Technol. 2016. PMID: 27505073 Free PMC article.
-
Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation.Ann N Y Acad Sci. 2014 Oct;1327(1):27-45. doi: 10.1111/nyas.12421. Epub 2014 Apr 14. Ann N Y Acad Sci. 2014. PMID: 24730978 Free PMC article. Review.
-
Advances in smoking cessation pharmacotherapy: Non-nicotinic approaches in animal models.Neuropharmacology. 2020 Nov 1;178:108225. doi: 10.1016/j.neuropharm.2020.108225. Epub 2020 Aug 3. Neuropharmacology. 2020. PMID: 32758566 Free PMC article. Review.
Cited by
-
The co-use of nicotine and prescription psychostimulants: A review of their behavioral and neuropharmacological interactions.Drug Alcohol Depend. 2023 Jul 1;248:109906. doi: 10.1016/j.drugalcdep.2023.109906. Epub 2023 May 4. Drug Alcohol Depend. 2023. PMID: 37216808 Free PMC article. Review.
-
From 2-Triethylammonium Ethyl Ether of 4-Stilbenol (MG624) to Selective Small-Molecule Antagonists of Human α9α10 Nicotinic Receptor by Modifications at the Ammonium Ethyl Residue.J Med Chem. 2022 Jul 28;65(14):10079-10097. doi: 10.1021/acs.jmedchem.2c00746. Epub 2022 Jul 14. J Med Chem. 2022. PMID: 35834819 Free PMC article.
-
An in vitro assay to investigate venom neurotoxin activity on muscle-type nicotinic acetylcholine receptor activation and for the discovery of toxin-inhibitory molecules.Biochem Pharmacol. 2023 Oct;216:115758. doi: 10.1016/j.bcp.2023.115758. Epub 2023 Aug 20. Biochem Pharmacol. 2023. PMID: 37604290 Free PMC article.
-
Subnanomolar Affinity and Selective Antagonism at α7 Nicotinic Receptor by Combined Modifications of 2-Triethylammonium Ethyl Ether of 4-Stilbenol (MG624).J Med Chem. 2023 Jan 12;66(1):306-332. doi: 10.1021/acs.jmedchem.2c01256. Epub 2022 Dec 16. J Med Chem. 2023. PMID: 36526469 Free PMC article.
References
-
- Babb S, Malarcher A, Schauer G, et al. Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65:1457–64. - PubMed
-
- National Center for Chronic Disease, P.; Health Promotion Office on, S.; Health. Reports of the Surgeon General. In The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta (GA), 2014. - PubMed
-
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci 2005;8:1465–70. - PubMed
-
- Lukas RJ, Changeux JP, Le Novere N, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 1999;51:397–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

