Biomarkers to identify Mycobacterium tuberculosis infection among borderline QuantiFERON results
- PMID: 35058249
- PMCID: PMC9363845
- DOI: 10.1183/13993003.02665-2021
Biomarkers to identify Mycobacterium tuberculosis infection among borderline QuantiFERON results
Abstract
Background: Screening for tuberculosis (TB) infection often includes QuantiFERON-TB Gold Plus (QFT) testing. Previous studies showed that two-thirds of patients with negative QFT results just below the cut-off, so-called borderline test results, nevertheless had other evidence of TB infection. This study aimed to identify a biomarker profile by which borderline QFT results due to TB infection can be distinguished from random test variation.
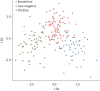
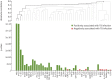
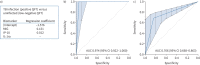
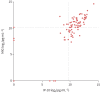
Methods: QFT supernatants of patients with a borderline (≥0.15 and <0.35 IU·mL-1), low-negative (<0.15 IU·mL-1) or positive (≥0.35 IU·mL-1) QFT result were collected in three hospitals. Bead-based multiplex assays were used to analyse 48 different cytokines, chemokines and growth factors. A prediction model was derived using LASSO regression and applied further to discriminate QFT-positive Mycobacterium tuberculosis-infected patients from borderline QFT patients and QFT-negative patients RESULTS: QFT samples of 195 patients were collected and analysed. Global testing revealed that the levels of 10 kDa interferon (IFN)-γ-induced protein (IP-10/CXCL10), monokine induced by IFN-γ (MIG/CXCL9) and interleukin-1 receptor antagonist in the antigen-stimulated tubes were each significantly higher in patients with a positive QFT result compared with low-negative QFT individuals (p<0.001). A prediction model based on IP-10 and MIG proved highly accurate in discriminating patients with a positive QFT (TB infection) from uninfected individuals with a low-negative QFT (sensitivity 1.00 (95% CI 0.79-1.00) and specificity 0.95 (95% CI 0.74-1.00)). This same model predicted TB infection in 68% of 87 patients with a borderline QFT result.
Conclusions: This study was able to classify borderline QFT results as likely infection-related or random. These findings support additional laboratory testing for either IP-10 or MIG following a borderline QFT result for individuals at increased risk of reactivation TB.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: T.H.M. Ottenhoff reports grants from NWO-TTW (PI: T.H.M. Ottenhoff), Dutch Government, Technical Sciences; ZonMw (PI: T.H.M. Ottenhoff), Dutch Government (ZonMw); IMI2 HOR2020 VSV EBOPLUS (PI: C.A. Siegrist), European Commission HOR2020 IMI2 Program; NWO-TTW (PI: J. Bouwstra), Dutch Government, Technical Sciences; NWO-TTW (PI: T.H.M. Ottenhoff), Dutch Government, Technical Sciences, NACTAR Program; NWO-Chemical Sciences (PI: A. Minnaard), Dutch Government, Technical Sciences; EC HOR2020 TRANSVAC2 (PI: European Vaccine Initiative (EVI)), European Commission HOR2020 Program; IMI2 EC HOR2020 Respiri-TB (PI: M. Lamers), European Commission HOR2020 IMI2 Program; IMI2 EC HOR2020 Respiri-NTM (PI: M. Lamers), European Commission HOR2020 IMI2 Program; NIH (PI: T.H.M. Ottenhoff); NIH, NIAID, grant: 1RO1AI141315-01A1; EC HOR2020 SMA-TB (PI: C. Vilaplana); European Commission HOR2020 Program; leadership at the Tuberculosis Vaccine Initiative (TBVI; www.tbvi.eu); outside the submitted work. S.A. Joosten reports grants from NIH (PI: T.H.M. Ottenhoff; co-PI: S.A. Joosten); NIH, NIAID, grant: 1RO1AI141315-01A1; outside the submitted work. S.M. Arend reports travel support from Oxford Immunotec, outside the submitted work. All other authors have nothing to disclose.
Figures


Comment in
-
Update June 2022: management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline.Eur Respir J. 2022 Aug 10;60(2):2200803. doi: 10.1183/13993003.00803-2022. Print 2022 Aug. Eur Respir J. 2022. PMID: 35710264 Free PMC article.
-
Improving interferon-γ release assay interpretation: are IP-10 and MIG the solution?Eur Respir J. 2022 Aug 10;60(2):2200697. doi: 10.1183/13993003.00697-2022. Print 2022 Aug. Eur Respir J. 2022. PMID: 35948350 No abstract available.
References
-
- Mazurek GH, Jereb J, Vernon A, et al. . Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep 2010; 59: 1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
