Checkpoint blockade-induced CD8+ T cell differentiation in head and neck cancer responders
- PMID: 35058328
- PMCID: PMC8772459
- DOI: 10.1136/jitc-2021-004034
Checkpoint blockade-induced CD8+ T cell differentiation in head and neck cancer responders
Abstract
Background: Immune checkpoint blockade (ICB) response in recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) is limited to 15%-20% of patients and underpinnings of resistance remain undefined.
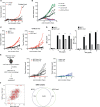
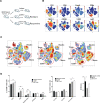
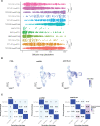
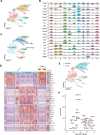
Methods: Starting with an anti-PD1 sensitive murine HNSCC cell line, we generated an isogenic anti-PD1 resistant model. Mass cytometry was used to delineate tumor microenvironments of both sensitive parental murine oral carcinoma (MOC1) and resistant MOC1esc1 tumors. To examine heterogeneity and clonal dynamics of tumor infiltrating lymphocytes (TILs), we applied paired single-cell RNA and TCR sequencing in three HNSCC models.
Results: Anti-PD1 resistant MOC1esc1 line displayed a conserved cell intrinsic immune evasion signature. Immunoprofiling showed distinct baseline tumor microenvironments of MOC1 and MOC1esc1, as well as the remodeling of immune compartments on ICB in MOC1esc1 tumors. Single cell sequencing analysis identified several CD8 +TIL subsets including Tcf7 +Pd1- (naïve/memory-like), Tcf7 +Pd1+ (progenitor), and Tcf7-Pd1+ (differentiated effector). Mapping TCR shared fractions identified that successful anti-PD1 or anti-CTLA4 therapy-induced higher post-treatment T cell lineage transitions.
Conclusions: These data highlight critical aspects of CD8 +TIL heterogeneity and differentiation and suggest facilitation of CD8 +TIL differentiation as a strategy to improve HNSCC ICB response.
Keywords: head and neck neoplasms; lymphocytes; tumor microenvironment; tumor-infiltrating.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RU serves on a Merck head and neck cancer advisory board. The MOC models developed by RU have been filed with the Washington University Office of Technology Management and are licensed for distribution by Kerafast. XSL is a cofounder, board member and Scientific Advisor of GV20 Oncotherapy, and on the Scientific Advisory Board of 3DMed Care. JS receives research support from Merck, BMS, Regeneron, Debiopharm, consulting/Scientific Advisory Board/travel fees from Genentech, Immunitas, Debiopharm, BMS, Nanobiotix, Tilos, AstraZeneca, LEK, Catenion, ACI Clinical, Astellas, Stimit., has stock options in Immunitas and equity in Doximity. RH consults for BMS, Merck, AstraZeneca, Pfizer, GSK, Genentech, Celgene, and Bayer, and received esearch Support from GSK, Merck, BMS, Pfizer, AstraZeneca, Genentech, and Kura.
Figures


Similar articles
-
Molecular patterns of resistance to immune checkpoint blockade in melanoma.Nat Commun. 2024 Apr 9;15(1):3075. doi: 10.1038/s41467-024-47425-y. Nat Commun. 2024. PMID: 38594286 Free PMC article.
-
Enhanced oral versus flank lymph node T cell response parallels anti-PD1 efficacy in head and neck cancer.Oral Oncol. 2024 May;152:106795. doi: 10.1016/j.oraloncology.2024.106795. Epub 2024 Apr 9. Oral Oncol. 2024. PMID: 38599127 Free PMC article.
-
Monocyte-derived APCs are central to the response of PD1 checkpoint blockade and provide a therapeutic target for combination therapy.J Immunother Cancer. 2020 Jul;8(2):e000588. doi: 10.1136/jitc-2020-000588. J Immunother Cancer. 2020. PMID: 32690667 Free PMC article.
-
Biomarkers and 3D models predicting response to immune checkpoint blockade in head and neck cancer (Review).Int J Oncol. 2022 Jul;61(1):88. doi: 10.3892/ijo.2022.5378. Epub 2022 Jun 1. Int J Oncol. 2022. PMID: 35642667 Free PMC article. Review.
-
Biomarkers of Immune Checkpoint Blockade Response in Triple-Negative Breast Cancer.Curr Treat Options Oncol. 2021 Mar 20;22(5):38. doi: 10.1007/s11864-021-00833-4. Curr Treat Options Oncol. 2021. PMID: 33743085 Review.
Cited by
-
New perspectives on biology, disease progression, and therapy response of head and neck cancer gained from single cell RNA sequencing and spatial transcriptomics.Oncol Res. 2023 Nov 15;32(1):1-17. doi: 10.32604/or.2023.044774. eCollection 2023. Oncol Res. 2023. PMID: 38188682 Free PMC article. Review.
-
The mouse oral carcinoma (MOC) model: A 10-year retrospective on model development and head and neck cancer investigations.Oral Oncol. 2022 Sep;132:106012. doi: 10.1016/j.oraloncology.2022.106012. Epub 2022 Jul 9. Oral Oncol. 2022. PMID: 35820346 Free PMC article. Review.
-
Morphine treatment restricts response to immunotherapy in oral squamous cell carcinoma.J Immunother Cancer. 2024 Nov 17;12(11):e009962. doi: 10.1136/jitc-2024-009962. J Immunother Cancer. 2024. PMID: 39551606 Free PMC article.
-
Mast Cell Infiltration and Subtype Promote Malignant Transformation of Oral Precancer and Progression of Oral Cancer.Cancer Res Commun. 2024 Aug 1;4(8):2203-2214. doi: 10.1158/2767-9764.CRC-24-0169. Cancer Res Commun. 2024. PMID: 39087378 Free PMC article.
-
Targeting Dendritic Cell Dysfunction to Circumvent Anti-PD1 Resistance in Head and Neck Cancer.Clin Cancer Res. 2024 May 1;30(9):1934-1944. doi: 10.1158/1078-0432.CCR-23-3477. Clin Cancer Res. 2024. PMID: 38372707 Free PMC article.
References
-
- Burtness B, Harrington KJ, Greil R, et al. . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. 10.1016/S0140-6736(19)32591-7 - DOI - PubMed
-
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. . Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. 10.1016/S0140-6736(16)00561-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
