Remodeling of lateral geniculate nucleus projections to extrastriate area MT following long-term lesions of striate cortex
- PMID: 35058366
- PMCID: PMC8794847
- DOI: 10.1073/pnas.2117137119
Remodeling of lateral geniculate nucleus projections to extrastriate area MT following long-term lesions of striate cortex
Abstract
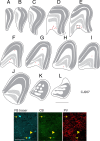
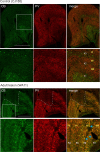
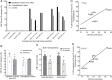
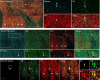
Here, we report on a previously unknown form of thalamocortical plasticity observed following lesions of the primary visual area (V1) in marmoset monkeys. In primates, lateral geniculate nucleus (LGN) neurons form parallel pathways to the cortex, which are characterized by the expression of different calcium-binding proteins. LGN projections to the middle temporal (MT) area only originate in the koniocellular layers, where many neurons express calbindin. In contrast, projections to V1 also originate in the magnocellular and parvocellular layers, where neurons express parvalbumin but not calbindin. Our results demonstrate that this specificity is disrupted following long-term (1 to 3 y) unilateral V1 lesions, indicating active rearrangement of the geniculocortical circuit. In lesioned animals, retrograde tracing revealed MT-projecting neurons scattered throughout the lesion projection zone (LPZ, the sector of the LGN that underwent retrograde degeneration following a V1 lesion). Many of the MT-projecting neurons had large cell bodies and were located outside the koniocellular layers. Furthermore, we found that a large percentage of magno- and parvocellular neurons expressed calbindin in addition to the expected parvalbumin expression and that this coexpression was present in many of the MT-projecting neurons within the LPZ. These results demonstrate that V1 lesions trigger neurochemical and structural remodeling of the geniculo-extrastriate pathway, leading to the emergence of nonkoniocellular input to MT. This has potential implications for our understanding of the neurobiological bases of the residual visual abilities that survive V1 lesions, including motion perception and blindsight, and reveals targets for rehabilitation strategies to ameliorate the consequences of cortical blindness.
Keywords: blindsight; brain plasticity; extrastriate cortex; primate; striate cortex lesion.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Horton J. C., Hoyt W. F., The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch. Ophthalmol. 109, 816–824 (1991). - PubMed
-
- Cowey A., Stoerig P., Projection patterns of surviving neurons in the dorsal lateral geniculate nucleus following discrete lesions of striate cortex: Implications for residual vision. Exp. Brain Res. 75, 631–638 (1989). - PubMed
-
- Atapour N., Worthy K. H., Lui L. L., Yu H. H., Rosa M. G. P., Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex: Comparison of young adult and geriatric marmoset monkeys. Brain Struct. Funct. 222, 3283–3293 (2017). - PubMed
-
- Atapour N., Worthy K. H., Rosa M. G. P., Neurochemical changes in the primate lateral geniculate nucleus following lesions of striate cortex in infancy and adulthood: Implications for residual vision and blindsight. Brain Struct. Funct. 226, 2763–2775 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

