A Female-Specific Role for Calcitonin Gene-Related Peptide (CGRP) in Rodent Pain Models
- PMID: 35058371
- PMCID: PMC8916765
- DOI: 10.1523/JNEUROSCI.1137-21.2022
A Female-Specific Role for Calcitonin Gene-Related Peptide (CGRP) in Rodent Pain Models
Abstract
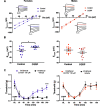
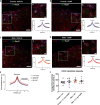
We aimed to investigate a sexually dimorphic role of calcitonin gene-related peptide (CGRP) in rodent models of pain. Based on findings in migraine where CGRP has a preferential pain-promoting effect in female rodents, we hypothesized that CGRP antagonists and antibodies would attenuate pain sensitization more efficaciously in female than male mice and rats. In hyperalgesic priming induced by activation of interleukin 6 signaling, CGRP receptor antagonists olcegepant and CGRP8-37 both given intrathecally, blocked, and reversed hyperalgesic priming only in females. A monoclonal antibody against CGRP, given systemically, blocked priming specifically in female rodents but failed to reverse it. In the spared nerve injury model, there was a transient effect of both CGRP antagonists, given intrathecally, on mechanical hypersensitivity in female mice only. Consistent with these findings, intrathecally applied CGRP caused a long-lasting, dose-dependent mechanical hypersensitivity in female mice but more transient effects in males. This CGRP-induced mechanical hypersensitivity was reversed by olcegepant and the KCC2 enhancer CLP257, suggesting a role for anionic plasticity in the dorsal horn in the pain-promoting effects of CGRP in females. In spinal dorsal horn slices, CGRP shifted GABAA reversal potentials to significantly more positive values, but, again, only in female mice. Therefore, CGRP may regulate KCC2 expression and/or activity downstream of CGRP receptors specifically in females. However, KCC2 hypofunction promotes mechanical pain hypersensitivity in both sexes because CLP257 alleviated hyperalgesic priming in male and female mice. We conclude that CGRP promotes pain plasticity in female rodents but has a limited impact in males.SIGNIFICANCE STATEMENT The majority of patients impacted by chronic pain are women. Mechanistic studies in rodents are creating a clear picture that molecular events promoting chronic pain are different in male and female animals. We sought to build on evidence showing that CGRP is a more potent and efficacious promoter of headache in female than in male rodents. To test this, we used hyperalgesic priming and the spared nerve injury neuropathic pain models in mice. Our findings show a clear sex dimorphism wherein CGRP promotes pain in female but not male mice, likely via a centrally mediated mechanism of action. Our work suggests that CGRP receptor antagonists could be tested for efficacy in women for a broader variety of pain conditions.
Keywords: CGRP; dorsal horn; hyperalgesic priming; neuropathic pain; olcegepant; pain.
Copyright © 2022 the authors.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
