Efficacy of adjuvant trastuzumab in women with HER2-positive T1a or bN0M0 breast cancer: a population-based cohort study
- PMID: 35058536
- PMCID: PMC8776836
- DOI: 10.1038/s41598-022-05209-8
Efficacy of adjuvant trastuzumab in women with HER2-positive T1a or bN0M0 breast cancer: a population-based cohort study
Abstract
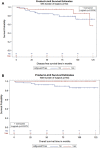
Adjuvant trastuzumab has been associated with superior survival in women with ≥ T1c or node-positive HER2-positive early-stage breast cancer; however, there is a lack of phase III trials in women with T1a/bN0 disease. Our study aimed to assess the outcomes of women with HER2-positive T1a/bN0 breast cancer who received adjuvant trastuzumab in Saskatchewan, Canada. We evaluated all women diagnosed with HER2-positive T1a/bN0 breast cancer in Saskatchewan between 2008 and 2017. We performed Cox proportional multivariable analysis to determine factors correlated with survival. In addition, inverse probability treatment weighting (IPTW) using propensity score was performed to assess benefit of adjuvant trastuzumab. Ninety-one eligible women with a median age of 61 years (range 30-89) were identified. Thirty-nine (43%) women received adjuvant trastuzumab. Women who received trastuzumab were younger and had a higher rate of T1b disease. Overall, 3% of women who received trastuzumab compared to 12% of women who did not receive trastuzumab developed breast cancer recurrence (p = 0.23). Five-year disease-free survival (DFS) of women who received adjuvant trastuzumab was 94.8% compared to 82.7% of women who did not receive trastuzumab (p = 0.22). Five-year overall survival was 100% of women who received trastuzumab compared to 90.4% of women who did not receive adjuvant trastuzumab (p = 0.038). In the multivariable analysis, grade III tumors were correlated with inferior DFS (hazard ratio [HR] 5.5, 95% CI [1.7-17.7]). The propensity score using the inverse probability of treatment weighting showed that lack of adjuvant trastuzumab was correlated inferior DFS, with an HR of 4 (95% CI 1.05-15.5). Women with HER2-positive T1a/bN0 breast cancer had overall low recurrence of breast cancer. However, the results of this exploratory analysis indicate that women who received adjuvant trastuzumab had better survival.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 2011;29(25):3366–3373. doi: 10.1200/JCO.2011.35.0868. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous