Karyopherin-mediated nucleocytoplasmic transport
- PMID: 35058649
- PMCID: PMC10101760
- DOI: 10.1038/s41580-021-00446-7
Karyopherin-mediated nucleocytoplasmic transport
Abstract
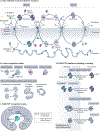
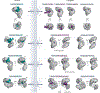
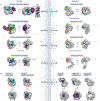
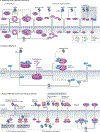
Efficient and regulated nucleocytoplasmic trafficking of macromolecules to the correct subcellular compartment is critical for proper functions of the eukaryotic cell. The majority of the macromolecular traffic across the nuclear pores is mediated by the Karyopherin-β (or Kap) family of nuclear transport receptors. Work over more than two decades has shed considerable light on how the different Kap family members bring their respective cargoes into the nucleus or the cytoplasm in efficient and highly regulated manners. In this Review, we overview the main features and established functions of Kap family members, describe how Kaps recognize their cargoes and discuss the different ways in which these Kap-cargo interactions can be regulated, highlighting new findings and open questions. We also describe current knowledge of the import and export of the components of three large gene expression machines - the core replisome, RNA polymerase II and the ribosome - pointing out the questions that persist about how such large macromolecular complexes are trafficked to serve their function in a designated subcellular location.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
Y.M.C. is a consultant for Faze Medicines. The remaining authors declare no competing interests.
Figures

References
-
- Matsuura Y Mechanistic insights from structural analyses of Ran-GTPase-driven nuclear export of proteins and RNAs. J. Mol. Biol 428, 2025–2039 (2016). - PubMed
-
- Kalita J, Kapinos LE & Lim RYH On the asymmetric partitioning of nucleocytoplasmic transport — recent insights and open questions. J. Cell Sci 134, jcs240382 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

