Effects of Sparganii Rhizoma on Osteoclast Formation and Osteoblast Differentiation and on an OVX-Induced Bone Loss Model
- PMID: 35058781
- PMCID: PMC8764242
- DOI: 10.3389/fphar.2021.797892
Effects of Sparganii Rhizoma on Osteoclast Formation and Osteoblast Differentiation and on an OVX-Induced Bone Loss Model
Abstract
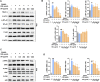
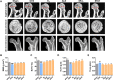
Postmenopausal osteoporosis is caused by an imbalance between osteoclasts and osteoblasts and causes severe bone loss. Osteoporotic medicines are classified into bone resorption inhibitors and bone formation promoters according to the mechanism of action. Long-term use of bisphosphonate and selective estrogen receptor modulators (SERMs) can cause severe side effects in postmenopausal osteoporosis patients. Therefore, it is important to find alternative natural products that reduce osteoclast activity and increase osteoblast formation. Sparganii Rhizoma (SR) is the dried tuberous rhizome of Sparganium stoloniferum Buchanan-Hamilton and is called "samreung" in Korea. However, to date, the effect of SR on osteoclast differentiation and the ovariectomized (OVX)-induced bone loss model has not been reported. In vitro, tartrate-resistant acid phosphatase (TRAP) staining, western blots, RT-PCR and other methods were used to examine the effect of SR on osteoclast differentiation and osteoblasts. In vivo, we confirmed the effect of SR in a model of OVX-induced postmenopausal osteoporosis. SR inhibited osteoclast differentiation and decreased the expression of TNF receptor-associated factor 6 (TRAF6), nuclear factor of activated T cells 1 (NFATc1) and c-Fos pathway. In addition, SR stimulates osteoblast differentiation and increased protein expression of the bone morphogenetic protein 2 (BMP-2)/SMAD signaling pathway. Moreover, SR protected against bone loss in OVX-induced rats. Our results appear to advance our knowledge of SR and successfully demonstrate its potential role as a osteoclastogenesis-inhibiting and osteogenesis-promoting herbal medicine for the treatment of postmenopausal osteoporosis.
Keywords: bone remodeling; osteoblast; osteoclast; ovariectomized; sparganii rhizoma.
Copyright © 2022 Lee, Kim, Hong, Kim, Kim, Sohn and Jung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











References
-
- Armstrong A. P., Tometsko M. E., Glaccum M., Sutherland C. L., Cosman D., Dougall W. C. (2002). A RANK/TRAF6-dependent Signal Transduction Pathway Is Essential for Osteoclast Cytoskeletal Organization and Resorptive Function. J. Biol. Chem. 277 (46), 44347–44356. 10.1074/jbc.M202009200 - DOI - PubMed
-
- Ballanti P., Minisola S., Pacitti M. T., Scarnecchia L., Rosso R., Mazzuoli G. F., et al. (1997). Tartrate-resistant Acid Phosphate Activity as Osteoclastic Marker: Sensitivity of Cytochemical Assessment and Serum Assay in Comparison with Standardized Osteoclast Histomorphometry. Osteoporos. Int. 7 (1), 39–43. 10.1007/BF01623458 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

