Use of Physiologically-Based Kinetics Modelling to Reliably Predict Internal Concentrations of the UV Filter, Homosalate, After Repeated Oral and Topical Application
- PMID: 35058784
- PMCID: PMC8763688
- DOI: 10.3389/fphar.2021.802514
Use of Physiologically-Based Kinetics Modelling to Reliably Predict Internal Concentrations of the UV Filter, Homosalate, After Repeated Oral and Topical Application
Abstract
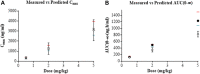
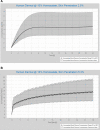
Ethical and legal considerations have led to increased use of non-animal methods to evaluate the safety of chemicals for human use. We describe the development and qualification of a physiologically-based kinetics (PBK) model for the cosmetic UV filter ingredient, homosalate, to support its safety without the need of generating further animal data. The intravenous (IV) rat PBK model, using PK-Sim®, was developed and validated using legacy in vivo data generated prior to the 2013 EU animal-testing ban. Input data included literature or predicted physicochemical and pharmacokinetic properties. The refined IV rat PBK model was subject to sensitivity analysis to identify homosalate-specific sensitive parameters impacting the prediction of Cmax (more sensitive than AUC(0-∞)). These were then considered, together with population modeling, to calculate the confidence interval (CI) 95% Cmax and AUC(0-∞). Final model parameters were established by visual inspection of the simulations and biological plausibility. The IV rat model was extrapolated to oral administration, and used to estimate internal exposures to doses tested in an oral repeated dose toxicity study. Next, a human PBK dermal model was developed using measured human in vitro ADME data and a module to represent the dermal route. Model performance was confirmed by comparing predicted and measured values from a US-FDA clinical trial (Identifier: NCT03582215, https://clinicaltrials.gov/). Final exposure estimations were obtained in a virtual population and considering the in vitro and input parameter uncertainty. This model was then used to estimate the Cmax and AUC(0-24 h) of homosalate according to consumer use in a sunscreen. The developed rat and human PBK models had a good biological basis and reproduced in vivo legacy rat and human clinical kinetics data. They also complied with the most recent WHO and OECD recommendations for assessing the confidence level. In conclusion, we have developed a PBK model which predicted reasonably well the internal exposure of homosalate according to different exposure scenarios with a medium to high level of confidence. In the absence of in vivo data, such human PBK models will be the heart of future completely non-animal risk assessments; therefore, valid approaches will be key in gaining their regulatory acceptance. Clinical Trial Registration: https://clinicaltrials.gov/, identifier, NCT03582215.
Keywords: UV filter; dermal application; homosalate; physiologically-based kinetics models; plasma concentration.
Copyright © 2022 Najjar, Schepky, Krueger, Dent, Cable, Li, Grégoire, Roussel, Noel-Voisin, Hewitt and Cardamone.
Conflict of interest statement
AN, AS, and C-TK are employed by Beiersdorf AG. MD, SC, and HL are employed by Unilever Safety and Environmental Assurance Centre. SG, LR, and AN-V are employed by L’Oréal. Each company use Homosalate in cosmetic products. Cosmetics Europe is the trade association for the cosmetics and personal care industry where author EC is employed and author NH provides services as a consultant. None of the authors will benefit financially from this work other than their salaries.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
Medical

