Protein conformational dynamics and phenotypic switching
- PMID: 35059032
- PMCID: PMC8724335
- DOI: 10.1007/s12551-021-00858-x
Protein conformational dynamics and phenotypic switching
Abstract
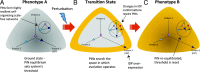
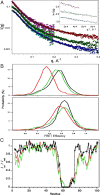
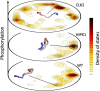
Intrinsically disordered proteins (IDPs) are proteins that lack rigid 3D structure but exist as conformational ensembles. Because of their structural plasticity, they can interact with multiple partners. The protein interactions between IDPs and their partners form scale-free protein interaction networks (PINs) that facilitate information flow in the cell. Because of their plasticity, IDPs typically occupy hub positions in cellular PINs. Furthermore, their conformational dynamics and propensity for post-translational modifications contribute to "conformational" noise which is distinct from the well-recognized transcriptional noise. Therefore, upregulation of IDPs in response to a specific input, such as stress, contributes to increased noise and, hence, an increase in stochastic, "promiscuous" interactions. These interactions lead to activation of latent pathways or can induce "rewiring" of the PIN to yield an optimal output underscoring the critical role of IDPs in regulating information flow. We have used PAGE4, a highly intrinsically disordered stress-response protein as a paradigm. Employing a variety of experimental and computational techniques, we have elucidated the role of PAGE4 in phenotypic switching of prostate cancer cells at a systems level. These cumulative studies over the past decade provide a conceptual framework to better understand how IDP conformational dynamics and conformational noise might facilitate cellular decision-making.
Keywords: Conformational noise; Intrinsically disordered proteins; MRK hypothesis; PAGE4; Phenotypic switching; Protein conformational dynamics.
© International Union for Pure and Applied Biophysics (IUPAB) and Springer-Verlag GmbH Germany, part of Springer Nature 2021.
Conflict of interest statement
Conflict of interest/Competing interestsThe authors declare no competing interests.
Figures



References
-
- Al Emran A, Marzese DM, Menon DR, Stark MS, Torrano J, Hammerlindl H, Zhang G, Brafford P, Salomon MP, Nelson N, Hammerlindl S, Gupta D, Mills GB, Lu Y, Sturm RA, Flaherty K, Hoon DSB, Gabrielli B, Herlyn M, Schaider H. Distinct histone modifications denote early stress-induced drug tolerance in cancer. Oncotarget. 2017;9(9):8206–8222. doi: 10.18632/oncotarget.23654. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

