Inactivators of Ornithine Aminotransferase for the Treatment of Hepatocellular Carcinoma
- PMID: 35059122
- PMCID: PMC8762738
- DOI: 10.1021/acsmedchemlett.1c00526
Inactivators of Ornithine Aminotransferase for the Treatment of Hepatocellular Carcinoma
Abstract
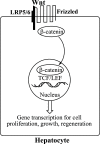
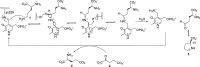
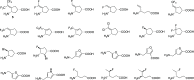
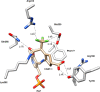
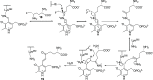
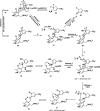
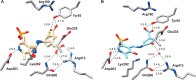
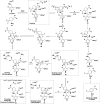
Hepatocellular carcinoma (HCC) is the second or third leading cause of cancer mortality worldwide (depending on which statistics are used), yet there is no effective treatment. Currently, there are nine FDA-approved drugs for HCC, five monoclonal antibodies and four tyrosine kinase inhibitors. Ornithine aminotransferase (OAT) has been validated as a target in preclinical studies, which demonstrates that it is a potential target to treat HCC. Currently, there are no OAT inactivators in clinical trials for HCC. This Innovation describes evidence to support inhibition of OAT as a novel approach for HCC tumor growth inhibition. After the mechanism of OAT is discussed, the origins of our involvement in OAT inactivation, based on our previous work on mechanism-based inactivation of GABA-AT, are described. Once it was demonstrated that OAT inactivation does lead to HCC tumor growth inhibition, new selective OAT inactivators were designed and their inactivation mechanisms were elucidated. A summary of these mechanistic studies is presented. Inactivators of OAT provide the potential for treatment of HCC, targeting the Wnt/β-catenin pathway.
© 2021 American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures





References
-
- Tang A.; Hallouch O.; Chernyak V.; Kamaya A.; Sirlin C. B. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom. Radiol. 2018, 43, 13–25. 10.1007/s00261-017-1209-1. - DOI - PubMed
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68, 394–424. 10.3322/caac.21492. - DOI - PubMed
- (c) https://www.bluefaery.org/statistics/.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

