Cu(i) catalysis for selective condensation/bicycloaromatization of two different arylalkynes: direct and general construction of functionalized C-N axial biaryl compounds
- PMID: 35059176
- PMCID: PMC8694356
- DOI: 10.1039/d1sc03865f
Cu(i) catalysis for selective condensation/bicycloaromatization of two different arylalkynes: direct and general construction of functionalized C-N axial biaryl compounds
Abstract
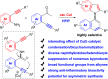
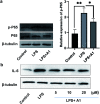
Selective condensation/bicycloaromatization of two different arylalkynes is firstly developed under ligand-free copper(i)-catalysis, which allows the direct synthesis of C-N axial biaryl compounds in high yields with excellent selectivity and functional group tolerance. Due to the critical effects of Cu(i) catalyst and HFIP, many easily occurring undesired reactions are suppressed, and the coupled five-six aromatic rings are constructed via the selective formation of two C(sp2)-N(sp2) bonds and four C(sp2)-C(sp2) bonds. The achievement of moderate enantioselectivity verifies its potential for the simplest asymmetric synthesis of atropoisomeric biaryls. Western blotting demonstrated that the newly developed compounds are promising targets in biology and pharmaceuticals. This unique reaction can construct structurally diverse C-N axial biaryl compounds that have never been reported by other methods, and might be extended to various applications in materials, chemistry, biology, and pharmaceuticals.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there are no conflicts to declare.
Figures






References
-
-
For selected books, see:
- Cepanec I., Synthesis of Biaryls, Elsevier, Amsterdam, Netherlands, 2004
- Larry Y., Privileged Structures in Drug Discovery, Wiley-VCH, Weinheim, Germany, 2018
- Burke A. J. and Marques C. S., Catalytic Arylation Methods-From the Academic Lab to Industrial Processes, Wiley-VCH, Weinheim, Germany, 2015
-
-
- Wezeman T. Brase S. Masters K. S. Nat. Prod. Rep. 2015;32:6–28. doi: 10.1039/C4NP00050A. - DOI - PubMed
- Huttel W. Muller M. Nat. Prod. Rep. 2021;38:1011–1043. doi: 10.1039/D0NP00010H. - DOI - PubMed
- Li Q. Xia T. Yao L. Deng H. Liao X. Chem. Sci. 2015;6:3599–3605. doi: 10.1039/C5SC00338E. - DOI - PMC - PubMed
- DeLorbe J. E. Horne D. Jove R. Mennen S. M. Nam S. Zhang F. L. Overman L. E. J. Am. Chem. Soc. 2013;135:4117–4128. doi: 10.1021/ja400315y. - DOI - PMC - PubMed
- Hu J. Sarrami F. Li H. Zhang G. Stubbs K. A. Lacey E. Stewart S. G. Karton A. Piggott A. M. Chooi Y. H. Chem. Sci. 2019;10:1457–1465. doi: 10.1039/C8SC02870B. - DOI - PMC - PubMed
- Podlesny E. E. Kozlowski M. C. J. Nat. Prod. 2012;75:1125–1129. doi: 10.1021/np300141t. - DOI - PMC - PubMed
-
- Du Y. L. Ryan K. S. Curr. Opin. Chem. Biol. 2016;31:74–81. doi: 10.1016/j.cbpa.2016.01.017. - DOI - PubMed
- Du Y. L. Ryan K. S. ACS Synth. Biol. 2015;4:682–688. doi: 10.1021/sb5003218. - DOI - PMC - PubMed
- Taha M. Uddin I. Gollapalli M. Almandil N. B. Rahim F. Farooq R. K. Nawaz M. Ibrahim M. Alqahtani M. A. Bamarouf Y. A. Selvaraj M. BMC Chem. 2019;13:102. doi: 10.1186/s13065-019-0617-4. - DOI - PMC - PubMed
- Arai T. Yamamoto Y. Awata A. Kamiya K. Ishibashi M. Arai M. A. Angew. Chem., Int. Ed. 2013;52:2486–2490. doi: 10.1002/anie.201208918. - DOI - PubMed
- Ren Y. Wang Y. Li G. Zhang Z. Ma L. Cheng B. Chen J. J. Med. Chem. 2021;64:4498–4515. doi: 10.1021/acs.jmedchem.0c01837. - DOI - PubMed
- Kaul M. Parhi A. K. Zhang Y. LaVoie E. J. Tuske S. Arnold E. Kerrigan J. E. Pilch D. S. J. Med. Chem. 2012;55:10160–10176. doi: 10.1021/jm3012728. - DOI - PMC - PubMed
-
- Wang H. Ji X. Li Z. Huang F. Adv. Mater. 2017;29:1606117. doi: 10.1002/adma.201606117. - DOI - PubMed
- Qian X. Shao L. Li H. Yan R. Wang X. Hou L. J. Power Sources. 2016;319:39–47. doi: 10.1016/j.jpowsour.2016.04.043. - DOI
- d'Ischia M. Napolitano A. Pezzella A. Meredith P. Sarna T. Angew. Chem., Int. Ed. 2009;48:3914–3921. doi: 10.1002/anie.200803786. - DOI - PMC - PubMed
- Xu X. Zhou X. Qu L. Wang L. Song J. Wu D. Zhou W. Zhou X. Xiang H. Wang J. Liu J. ACS Appl. Bio Mater. 2020;3:7236–7242. doi: 10.1021/acsabm.0c01063. - DOI - PubMed
LinkOut - more resources
Full Text Sources

