Using crystallography tools to improve vaccine formulations
- PMID: 35059205
- PMCID: PMC8733884
- DOI: 10.1107/S205225252101071X
Using crystallography tools to improve vaccine formulations
Abstract
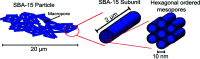
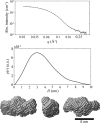
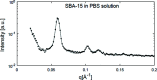
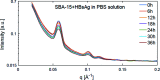
This article summarizes developments attained in oral vaccine formulations based on the encapsulation of antigen proteins inside porous silica matrices. These vaccine vehicles show great efficacy in protecting the proteins from the harsh acidic stomach medium, allowing the Peyer's patches in the small intestine to be reached and consequently enhancing immunity. Focusing on the pioneering research conducted at the Butantan Institute in Brazil, the optimization of the antigen encapsulation yield is reported, as well as their distribution inside the meso- and macroporous network of the porous silica. As the development of vaccines requires proper inclusion of antigens in the antibody cells, X-ray crystallography is one of the most commonly used techniques to unveil the structure of antibody-combining sites with protein antigens. Thus structural characterization and modelling of pure antigen structures, showing different dimensions, as well as their complexes, such as silica with encapsulated hepatitis B virus-like particles and diphtheria anatoxin, were performed using small-angle X-ray scattering, X-ray absorption spectroscopy, X-ray phase contrast tomography, and neutron and X-ray imaging. By combining crystallography with dynamic light scattering and transmission electron microscopy, a clearer picture of the proposed vaccine complexes is shown. Additionally, the stability of the immunogenic complex at different pH values and temperatures was checked and the efficacy of the proposed oral immunogenic complex was demonstrated. The latter was obtained by comparing the antibodies in mice with variable high and low antibody responses.
Keywords: SAXS; XAS; imaging; oral vaccines; porous silica.
© Márcia Carvalho de Abreu Fantini et al. 2022.
Figures





References
-
- Calderón, M. & Sosnik, A. (2015). Biotechnol. Adv. 33, 1277–1278. - PubMed
-
- Carvalho, L. V., Ruiz, R. D., Scaramuzzi, K., Marengo, E. B., Matos, J. R., Tambourgi, D. V., Fantini, M. C. A. & Sant’Anna, O. A. (2010). Vaccine, 28, 7829–7836. - PubMed
-
- Chen, L. J., Liu, J., Zhang, Y. L., Zhang, G. L., Kang, Y. Y., Chen, A. J., Feng, X. & Shao, L. Q. (2018). Nanomedicine, 13, 1939–1962. - PubMed
-
- Dobrovolskaia, M. A., Germolec, D. R. & Weaver, J. L. (2009). Nat. Nanotech. 4, 411–414. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

