Differences in thermal expansion and motion ability for herringbone and face-to-face π-stacked solids
- PMID: 35059207
- PMCID: PMC8733877
- DOI: 10.1107/S2052252521009593
Differences in thermal expansion and motion ability for herringbone and face-to-face π-stacked solids
Abstract
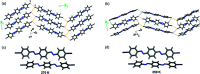
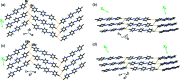
A series of aromatic organic molecules functionalized with different halogen atoms (I/ Br), motion-capable groups (olefin, azo or imine) and molecular length were designed and synthesized. The molecules self-assemble in the solid state through halogen bonding and exhibit molecular packing sustained by either herringbone or face-to-face π-stacking, two common motifs in organic semiconductor molecules. Interestingly, dynamic pedal motion is only achieved in solids with herringbone packing. On average, solids with herringbone packing exhibit larger thermal expansion within the halogen-bonded sheets due to motion occurrence and molecular twisting, whereas molecules with face-to-face π-stacking do not undergo motion or twisting. Thermal expansion along the π-stacked direction is surprisingly similar, but slightly larger for the face-to-face π-stacked solids due to larger changes in π-stacking distances with temperature changes. The results speak to the importance of crystal packing and intermolecular interaction strength when designing aromatic-based solids for organic electronics applications.
Keywords: crystal engineering; halogen bonding; intermolecular interactions; pedal motion; properties of solids; thermal expansion; π-stacking.
© Xiaodan Ding et al. 2022.
Figures










References
-
- Amit, A. G. & Hope, H. (1966). Acta Chem. Scand. 20, 835–844.
-
- Ashokkumar, S., Veeramani, V., Kaliannan, T., Stoeckli-Evans, H., Philip, R., Rose, P., Ramamurthi, K. & Babu, R. R. (2021). CSD Conmunication (CCDC deposition number 1583365). CCDC, Cambridge, England.
-
- Bernstein, L. & Izak, I. (1975). J. Cryst. Mol. Struct. 5, 257–266.
-
- Bhattacharya, S. & Saha, B. K. (2014). CrystEngComm, 16, 2340–2343.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

