Cantaloupe melon genome reveals 3D chromatin features and structural relationship with the ancestral cucurbitaceae karyotype
- PMID: 35059606
- PMCID: PMC8760558
- DOI: 10.1016/j.isci.2021.103696
Cantaloupe melon genome reveals 3D chromatin features and structural relationship with the ancestral cucurbitaceae karyotype
Abstract
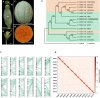
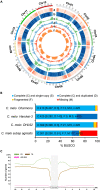
Cucumis melo displays a large diversity of horticultural groups with cantaloupe melon the most cultivated type. Using a combination of single-molecule sequencing, 10X Genomics link-reads, high-density optical and genetic maps, and chromosome conformation capture (Hi-C), we assembled a chromosome scale C. melo var. cantalupensis Charentais mono genome. Integration of RNA-seq, MeDip-seq, ChIP-seq, and Hi-C data revealed a widespread compartmentalization of the melon genome, segregating constitutive heterochromatin and euchromatin. Genome-wide comparative and evolutionary analysis between melon botanical groups identified Charentais mono genome increasingly more divergent from Harukei-3 (reticulatus), Payzawat (inodorus), and HS (ssp. agrestis) genomes. To assess the paleohistory of the Cucurbitaceae, we reconstructed the ancestral Cucurbitaceae karyotype and compared it to sequenced cucurbit genomes. In contrast to other species that experienced massive chromosome shuffling, melon has retained the ancestral genome structure. We provide comprehensive genomic resources and new insights in the diversity of melon horticultural groups and evolution of cucurbits.
Keywords: genomics; omics; plant biology; plant evolution.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A., Jordan T., Shakir K., Roazen D., Thibault J., et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics. 2013;43:1–33. doi: 10.1002/0471250953.bi1110s43. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

