Structural equation modelling the relationship between anti-fungal prophylaxis and Pseudomonas bacteremia in ICU patients
- PMID: 35059904
- PMCID: PMC8776977
- DOI: 10.1186/s40635-022-00429-8
Structural equation modelling the relationship between anti-fungal prophylaxis and Pseudomonas bacteremia in ICU patients
Abstract
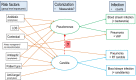
Purpose: Animal models implicate candida colonization facilitating invasive bacterial infections. The clinical relevance of this microbial interaction remains undefined and difficult to study directly. Observations from studies of anti-septic, antibiotic, anti-fungal, and non-decontamination-based interventions to prevent ICU acquired infection collectively serve as a natural experiment.
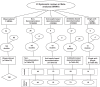
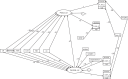
Methods: Three candidate generalized structural equation models (GSEM), with Candida and Pseudomonas colonization as latent variables, were confronted with blood culture and respiratory tract isolate data derived from 464 groups from 279 studies including studies of combined antibiotic and antifungal exposures within selective digestive decontamination (SDD) interventions.
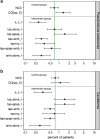
Results: Introducing an interaction term between Candida colonization and Pseudomonas colonization substantially improved GSEM model fit. Model derived coefficients for singular exposure to anti-septic agents (- 1.23; - 2.1 to - 0.32), amphotericin (- 1.78; - 2.79 to - 0.78) and topical antibiotic prophylaxis (TAP; + 1.02; + 0.11 to + 1.93) versus Candida colonization were similar in magnitude but contrary in direction. By contrast, the model-derived coefficients for singular exposure to TAP, as with anti-septic agents, versus Pseudomonas colonization were weaker or non-significant. Singular exposure to amphotericin would be predicted to more than halve candidemia and Pseudomonas bacteremia incidences versus literature benchmarks for absolute differences of approximately one percentage point or less.
Conclusion: GSEM modelling of published data supports the postulated interaction between Candida and Pseudomonas colonization towards promoting bacteremia among ICU patients. This would be difficult to detect without GSEM modelling. The model indicates that anti-fungal agents have greater impact in preventing Pseudomonas bacteremia than TAP, which has no impact.
Keywords: Anti-fungal; Candidemia; Generalized structural equation modelling; Pseudomonas bacteremia; Topical antibiotics.
© 2022. The Author(s).
Conflict of interest statement
The author declares that he has no competing interests.
Figures

Similar articles
-
Candida and the Gram-positive trio: testing the vibe in the ICU patient microbiome using structural equation modelling of literature derived data.Emerg Themes Epidemiol. 2022 Aug 18;19(1):7. doi: 10.1186/s12982-022-00116-9. Emerg Themes Epidemiol. 2022. PMID: 35982466 Free PMC article.
-
Staphylococcus aureus hitchhiking from colonization to bacteremia via Candida within ICU infection prevention studies: a proof of concept modelling.Eur J Clin Microbiol Infect Dis. 2023 May;42(5):543-554. doi: 10.1007/s10096-023-04573-1. Epub 2023 Mar 6. Eur J Clin Microbiol Infect Dis. 2023. PMID: 36877261 Free PMC article.
-
Candida-Acinetobacter-Pseudomonas Interaction Modelled within 286 ICU Infection Prevention Studies.J Fungi (Basel). 2020 Oct 27;6(4):252. doi: 10.3390/jof6040252. J Fungi (Basel). 2020. PMID: 33121074 Free PMC article.
-
Structural equation modeling the "control of gut overgrowth" in the prevention of ICU-acquired Gram-negative infection.Crit Care. 2020 May 4;24(1):189. doi: 10.1186/s13054-020-02906-6. Crit Care. 2020. PMID: 32366267 Free PMC article.
-
Structural Equation Modelling as a Proof-of-Concept Tool for Mediation Mechanisms Between Topical Antibiotic Prophylaxis and Six Types of Blood Stream Infection Among ICU Patients.Antibiotics (Basel). 2024 Nov 18;13(11):1096. doi: 10.3390/antibiotics13111096. Antibiotics (Basel). 2024. PMID: 39596789 Free PMC article. Review.
Cited by
-
Establishing the safety of selective digestive decontamination within the ICU population: a bridge too far?Trials. 2023 May 17;24(1):337. doi: 10.1186/s13063-023-07356-3. Trials. 2023. PMID: 37198636 Free PMC article.
-
Candida and the Gram-positive trio: testing the vibe in the ICU patient microbiome using structural equation modelling of literature derived data.Emerg Themes Epidemiol. 2022 Aug 18;19(1):7. doi: 10.1186/s12982-022-00116-9. Emerg Themes Epidemiol. 2022. PMID: 35982466 Free PMC article.
-
Staphylococcus aureus hitchhiking from colonization to bacteremia via Candida within ICU infection prevention studies: a proof of concept modelling.Eur J Clin Microbiol Infect Dis. 2023 May;42(5):543-554. doi: 10.1007/s10096-023-04573-1. Epub 2023 Mar 6. Eur J Clin Microbiol Infect Dis. 2023. PMID: 36877261 Free PMC article.
-
Indirect (herd) effects of topical antibiotic prophylaxis and oral care versus non-antimicrobial methods increase mortality among ICU patients: realigning Cochrane review data to emulate a three-tier cluster randomised trial.BMJ Open. 2023 Nov 30;13(11):e064256. doi: 10.1136/bmjopen-2022-064256. BMJ Open. 2023. PMID: 38035749 Free PMC article. Review.
References
-
- Albert M, Williamson D, Muscedere J, Lauzier F, Rotstein C, Kanji S, et al. Candida in the respiratory tract secretions of critically ill patients and the impact of antifungal treatment: a randomized placebo controlled pilot trial (CANTREAT study) Intensive Care Med. 2014;40:1313–1322. doi: 10.1007/s00134-014-3352-2. - DOI - PubMed
-
- Timsit JF, Schwebel C, Styfalova L, Cornet M, Poirier P, Forrestier C, et al. Impact of bronchial colonization with Candida spp. on the risk of bacterial ventilator-associated pneumonia in the ICU: the FUNGIBACT prospective cohort study. Intensive Care Med. 2019;45(6):834–843. doi: 10.1007/s00134-019-05622-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

