Quantifying regional α -synuclein, amyloid β, and tau accumulation in lewy body dementia
- PMID: 35060360
- PMCID: PMC8862415
- DOI: 10.1002/acn3.51482
Quantifying regional α -synuclein, amyloid β, and tau accumulation in lewy body dementia
Abstract
Objective: Parkinson disease (PD) is defined by the accumulation of misfolded α-synuclein (α-syn) in Lewy bodies and Lewy neurites. It affects multiple cortical and subcortical neuronal populations. The majority of people with PD develop dementia, which is associated with Lewy bodies in neocortex and referred to as Lewy body dementia (LBD). Other neuropathologic changes, including amyloid β (Aβ) and tau accumulation, occur in some LBD cases. We sought to quantify α-syn, Aβ, and tau accumulation in neocortical, limbic, and basal ganglia regions.
Methods: We isolated insoluble protein from fresh frozen postmortem brain tissue samples for eight brains regions from 15 LBD, seven Alzheimer disease (AD), and six control cases. We measured insoluble α-syn, Aβ, and tau with recently developed sandwich ELISAs.
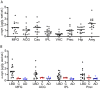
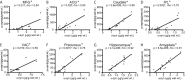
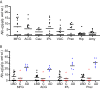
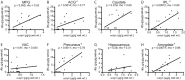
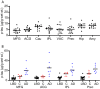
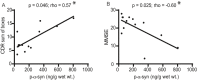
Results: We detected a wide range of insoluble α-syn accumulation in LBD cases. The majority had substantial α-syn accumulation in most regions, and dementia severity correlated with neocortical α-syn. However, three cases had low neocortical levels that were indistinguishable from controls. Eight LBD cases had substantial Aβ accumulation, although the mean Aβ level in LBD was lower than in AD. The presence of Aβ was associated with greater α-syn accumulation. Tau accumulation accompanied Aβ in only one LBD case.
Interpretation: LBD is associated with insoluble α-syn accumulation in neocortical regions, but the relatively low neocortical levels in some cases suggest that other changes contribute to impaired function, such as loss of neocortical innervation from subcortical regions. The correlation between Aβ and α-syn accumulation suggests a pathophysiologic relationship between these two processes.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Figures


References
-
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55(3):259‐272. - PubMed
-
- Appel‐Cresswell S, Vilarino‐Guell C, Encarnacion M, et al. Alpha‐synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov Disord. 2013;28(6):811‐813. - PubMed
-
- Golbe LI, Di Iorio G, Bonavita V, Miller DC, Duvoisin RC. A large kindred with autosomal dominant Parkinson's disease. Ann Neurol. 1990;27(3):276‐282. - PubMed
-
- Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha‐synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106‐108. - PubMed
-
- Lesage S, Anheim M, Letournel F, et al. G51D alpha‐synuclein mutation causes a novel parkinsonian‐pyramidal syndrome. Ann Neurol. 2013;73(4):459‐471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

