Oral antihistamine-decongestant-analgesic combinations for the common cold
- PMID: 35060618
- PMCID: PMC8780136
- DOI: 10.1002/14651858.CD004976.pub4
Oral antihistamine-decongestant-analgesic combinations for the common cold
Abstract
Background: Although combination formulas containing antihistamines, decongestants, and/or analgesics are sold over-the-counter in large quantities for the common cold, the evidence for their effectiveness is limited. This is an update of a review first published in 2012.
Objectives: To assess the effectiveness of antihistamine-decongestant-analgesic combinations compared with placebo or other active controls (excluding antibiotics) in reducing the duration of symptoms and alleviating symptoms (general feeling of illness, nasal congestion, rhinorrhoea, sneezing, and cough) in children and adults with the common cold.
Search methods: We searched CENTRAL, MEDLINE via EBSCOhost, Embase, CINAHL via EBSCOhost, LILACS, and Web of Science to 10 June 2021. We searched the WHO ICTRP and ClinicalTrials.gov on 10 June 2021.
Selection criteria: Randomised controlled trials investigating the effectiveness of antihistamine-decongestant-analgesic combinations compared with placebo, other active treatment (excluding antibiotics), or no treatment in children and adults with the common cold.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We assessed the certainty of the evidence using the GRADE approach. We categorised the included trials according to the active ingredients.
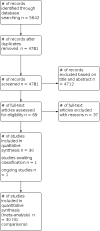
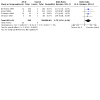
Main results: We identified 30 studies (6304 participants) including 31 treatment comparisons. The control intervention was placebo in 26 trials and an active substance (paracetamol, chlorphenindione + phenylpropanolamine + belladonna, diphenhydramine) in six trials (two trials had placebo as well as active treatment arms). Reporting of methods was generally poor, and there were large differences in study design, participants, interventions, and outcomes. Most of the included trials involved adult participants. Children were included in nine trials. Three trials included very young children (from six months to five years), and five trials included children aged 2 to 16. One trial included adults and children aged 12 years or older. The trials took place in different settings: university clinics, paediatric departments, family medicine departments, and general practice surgeries. Antihistamine-decongestant: 14 trials (1298 participants). Eight trials reported on global effectiveness, of which six studies were pooled (281 participants on active treatment and 284 participants on placebo). The odds ratio (OR) of treatment failure was 0.31 (95% confidence interval (CI) 0.20 to 0.48; moderate certainty evidence); number needed to treat for an additional beneficial outcome (NNTB) 3.9 (95% CI 3.03 to 5.2). On the final evaluation day (follow-up: 3 to 10 days), 55% of participants in the placebo group had a favourable response compared to 70% on active treatment. Of the two trials not pooled, one showed some global effect, whilst the other showed no effect. Adverse effects: the antihistamine-decongestant group experienced more adverse effects than the control group: 128/419 (31%) versus 100/423 (13%) participants suffered one or more adverse effects (OR 1.58, 95%CI 0.78 to 3.21; moderate certainty of evidence). Antihistamine-analgesic: four trials (1608 participants). Two trials reported on global effectiveness; data from one trial were presented (290 participants on active treatment and 292 participants on ascorbic acid). The OR of treatment failure was 0.33 (95% CI 0.23 to 0.46; moderate certainty evidence); NNTB 6.67 (95% CI 4.76 to 12.5). Forty-three per cent of participants in the control group and 70% in the active treatment group were cured after six days of treatment. The second trial also showed an effect in favour of the active treatment. Adverse effects: there were not significantly more adverse effects in the active treatment group compared to placebo (drowsiness, hypersomnia, sleepiness 10/152 versus 4/120; OR 1.64 (95 % CI 0.48 to 5.59; low certainty evidence). Analgesic-decongestant: seven trials (2575 participants). One trial reported on global effectiveness: 73% of participants in the analgesic-decongestant group reported a benefit compared with 52% in the control group (paracetamol) (OR of treatment failure 0.28, 95% CI 0.15 to 0.52; moderate certainty evidence; NNTB 4.7). Adverse effects: the decongestant-analgesic group experienced significantly more adverse effects than the control group (199/1122 versus 75/675; OR 1.62 95% CI 1.18 to 2.23; high certainty evidence; number needed to treat for an additional harmful outcome (NNTH 17). Antihistamine-analgesic-decongestant: six trials (1014 participants). Five trials reported on global effectiveness, of which two studies in adults could be pooled: global effect reported with active treatment (52%) and placebo (34%) was equivalent to a difference of less than one point on a four- or five-point scale; the OR of treatment failure was 0.47 (95% CI 0.33 to 0.67; low certainty evidence); NNTB 5.6 (95% CI 3.8 to 10.2). One trial in children aged 2 to 12 years, and two trials in adults found no beneficial effect. Adverse effects: in one trial 5/224 (2%) suffered adverse effects with the active treatment versus 9/208 (4%) with placebo. Two other trials reported no differences between treatment groups.
Authors' conclusions: We found a lack of data on the effectiveness of antihistamine-analgesic-decongestant combinations for the common cold. Based on these scarce data, the effect on individual symptoms is probably too small to be clinically relevant. The current evidence suggests that antihistamine-analgesic-decongestant combinations have some general benefit in adults and older children. These benefits must be weighed against the risk of adverse effects. There is no evidence of effectiveness in young children. In 2005, the US Food and Drug Administration issued a warning about adverse effects associated with the use of over-the-counter nasal preparations containing phenylpropanolamine.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
An IM De Sutter has declared that they have no conflict of interest. Lars Eriksson has declared that they have no conflict of interest. Mieke L van Driel has declared that they have no conflict of interest. Anna Gomez has declared that they have no conflict of interest.
Figures

















Update of
-
Oral antihistamine-decongestant-analgesic combinations for the common cold.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD004976. doi: 10.1002/14651858.CD004976.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2022 Jan 21;1:CD004976. doi: 10.1002/14651858.CD004976.pub4. PMID: 22336807 Updated.
Comment in
-
Oral Antihistamine/Analgesic/Decongestant Combinations for the Common Cold.Am Fam Physician. 2022 Sep;106(3):248-249. Am Fam Physician. 2022. PMID: 36126004 No abstract available.
References
References to studies included in this review
Aschan 1974 {published data only}
-
- Aschan G.Decongestion of nasal mucous membranes by oral medication in acute rhinitis. A rhinomanometric study to demonstrate synergism between antihistamines and adrenergic substance. Acta Otolaryngologica 1974;77(6):433-8. - PubMed
Berkowitz 1989 {published data only}
-
- Berkowitz RB, Connell JT, Dietz AJ, Greenstein SM, Tinkelman DG.The effectiveness of the non sedating antihistamine loratadine plus pseudoephedrine in the symptomatic management of the common cold. Annals of Allergy 1989;63(4):336-9. - PubMed
Blanco 2000 {published data only}
-
- Blanco de la Mora E, Cardillo L, De la Barrera, Marky B.Efficacy and safety of loratadine, pseudoephedrine and acetaminophen in the non-sedating symptomatic treatment of the common cold. Investigacion Medica Internacional 2000;24:14-25.
Bye 1980 {published data only}
Clemens 1997 {published data only}
Curley 1988 {published data only}
-
- Curley FJ, Irwin RS, Pratter MR, Stivers DH, Doern GV, Vernaglia PA, et al.Cough and the common cold. American Review of Respiratory Disease 1988;138(2):305-11. - PubMed
Debelic 1973 {published data only}
-
- Debelic M, Radielovic P.Therapy of banal rhinitis. Double-blind controlled clinical study. Schweizerische Rundschau fur Medizin Praxis 1973;62(35):1074-7. - PubMed
Eccles 2006a {published data only}
-
- Eccles R, Jawad M, Jawad S, Ridge D, North M, Jones E, et al.Efficacy of a paracetamol-pseudoephedrine combination for treatment of nasal congestion and pain-related symptoms in upper respiratory tract infection. Current Medical Research 2006;22(12):2411-8. - PubMed
Eccles 2014 {published data only}
Galvez 1985 {published data only}
-
- Galvez J.Symptomatic treatment of patients with the common cold. Clinical Trials Journal 1985;22(6):489-97.
Gwaltney 2002 {published data only}
Hutton 1991 {published data only}
-
- Hutton N, Wilson MH, Mellits ED, Baumgardner R, Wissow LS, Bonuccelli C, et al.Effectiveness of an antihistamine-decongestant combination for young children with the common cold: a randomized, controlled clinical trial. Journal of Pediatrics 1991;118(1):125-30. - PubMed
Koytchev 2003 {published data only}
-
- Koytchev R, Vlahov V, Bacratcheva N, Giesel B, Gawronska-Szklarz B, Wojcicki J, et al.Evaluation of the efficacy of a combined formulation (Grippostad-C) in the therapy of symptoms of common cold: a randomized, double-blind, multicenter trial. International Journal of Clinical Pharmacology and Therapeutics 2003;41(3):114-25. - PubMed
Lebacq 1994 {published data only}
-
- Lebacq E Jr, Gline JP, Joue P, Stockis A, Calderon P, Van Schoor O, et al.Exploratory study of the decongestive effect of Rhinopront syrup in adults and in children with acute rhinitis. Clinical Trials and Meta-analysis 1994;29(2-3):113-24. - PubMed
Loose 2004 {published data only}
-
- Loose I, Winkel M.Clinical, double-blind, placebo-controlled study investigating the combination of acetylsalicylic acid and pseudoephedrine for the symptomatic treatment of nasal congestion associated with common cold. Arzneimittelforschung 2004;54(9):513-21. - PubMed
Martinez 1994 {published data only}
-
- Martinez Gallardo F, López Fiesco A, Zamora G.Symptomatic treatment of common cold in children with a new combination of naproxen sodium plus pseudoephedrine hydrochloride: a comparative trial against pseudoephedrine syrup. Proceedings of the Western Pharmacology Society 1994;37:157-8. - PubMed
Middleton 1981 {published data only}
-
- Middleton RS.Double blind trial in general practice comparing the efficacy of "Benylin Day and Night" and paracetamol in the treatment of the common cold. British Journal of Clinical Practice 1981;35(9):297-300. - PubMed
Mizoguchi 2007 {published data only}
-
- Mizoguchi H, Wilson A, Jerdack GR, Hulle JD, Goodale M, Grender JM, et al.Efficacy of a single evening dose of a syrup containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulfate in subjects with multiple common cold symptoms. International Journal of Clinical Pharmacology and Therapeutics 2007;45(4):230-6. - PubMed
Montijo‐Barrios 2011 {published data only}
-
- Montijo-Barrios E, Cadena F, Ramirez-Mayans JA, Gutierrez-Castellon P.Clinical trial on the effect of buphenine, aminophenazone and diphenylpyraline hydrochloride in treating the common cold in children of 6 to 24 months of age [Ensayo clínico sobre el efecto de la bufenina, aminofenazona y el clorhidrato de difenilpiralina en el tratamiento del resfriado común en niños de seis a 24 meses de edad]. Revista de Investigacion Clinica 2011;63:335-43. - PubMed
Robert 2004 {published data only}
-
- Common Cold Collaborative Group, Robert M, Llorens M, Garcia E, Luria X.Efficacy and tolerability of ebastine 10 mg plus pseudoephedrine 120 mg in the symptomatic relief of the common cold. European Journal of Internal Medicine 2004;15(4):242-7. - PubMed
Sachsenroder 1972 {published data only}
-
- Sachsenroder L, Renovanz HD, Flach D.Efficacy and tolerance of a combined oral rhinologic agent. Arzneimittelforschung 1972;22(2):392-8. - PubMed
Scavino 1985 {published data only}
-
- Scavino Y.Combination therapy in patients with the common cold. Current Therapeutic Research 1985;38(5):746-54.
Schrooten 1993 {published data only}
-
- Schrooten P, Laekeman G, Vos P, De Munck G.Community pharmacists as clinical investigators in the self medication area: a double-blind, placebo-controlled study with astemizole-D in the common cold. International Pharmacy Journal 1993;7:147-50.
Sperber 1989 {published data only}
Sperber 2000 {published data only}
-
- Sperber SJ, Turner RB, Sorrentino JV, O'Connor RR, Rogers J, Gwaltney JM Jr.Effectiveness of pseudoephedrine plus acetaminophen for treatment of symptoms attributed to the paranasal sinuses associated with the common cold. Archives of Family Medicine 2000;9(10):979-85. - PubMed
Thackray 1978 {published data only}
-
- Thackray P.A double-blind, crossover controlled evaluation of a syrup for the night-time relief of the symptoms of the common cold, containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulphate. Journal of Internal Medicine 1978;6(2):161-5. - PubMed
Unuvar 2007 {published data only}
-
- Unuvar E, Yildiz I, Kilic A, Toprak S, Selvi Aslan S, Aydin S, et al.Is acetaminophen as effective as an antihistamine-decongestant-acetaminophen combination in relieving symptoms of acute nasopharyngitis in children? A randomised, controlled trial. International Journal of Pediatric Otorhinolaryngology 2007;71(8):1277-85. - PubMed
Virtanen 1983 {published data only}
-
- Virtanen H.A slow release combined preparation (dexchlorpheniramine + pseudoephedrine) for symptomatic treatment of the common cold. Journal of Laryngology and Otology 1983;97(2):159-63. - PubMed
Weippl 1984 {published data only}
-
- Weippl G.Therapeutic approaches to the common cold in children. Clinical Therapeutics 1984;6(4):475-82. - PubMed
Zhang 2018 {published data only}
-
- Zhang Y, Mallefet P.Time-to-onset of cold and flu symptom relief: a randomized, double-blind, placebo controlled pilot study for a multi-symptom combination product. International Journal of Clinical Pharmacology and Therapeutics 2018;56(12):604-11. - PubMed
References to studies excluded from this review
Axelsson 1971 {published data only}
-
- Axelsson A, Hammer G.Treatment of vasomotor rhinitis with a combined antihistaminic-sympathomimetic preparation. Acta Allergologica 1971;26(5):357-62. - PubMed
Bachert 2005 {published data only}
-
- Bachert C, Chuchalin AG, Eisebitt R, Netayazhenko VZ, Voelker M.Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clinical Therapeutics 2005;27(7):993-1003. - PubMed
Bhattacharya 2013 {published data only}
Bonifaci 1977 {published data only}
-
- Bonifaci E, Giorgi-Conciato M, Vibelli C.Symptomatic treatment of acute inflammation of the upper respiratory airways in pediatrics. Minerva Pediatrica 1970;29(5):285-96. - PubMed
Cantekin 1980 {published data only}
-
- Cantekin EI, Bluestone CD, Rockette HE, Beery QC.Effect of decongestant with or without antihistamine on Eustachian tube function. Annals of Otology, Rhinology, and Laryngology Supplement 1980;89(3 Pt 2):290-5. - PubMed
Carta 1967 {published data only}
-
- Carta F.Clinical experience with the vasoconstrictor action of H 1032 on the nasal mucosa. Archivio Italiano di Otologia, Rinologia-Laringologia e Patologia Cervico-facciale 1967;78(4):510-9. - PubMed
Chung 1991 {published data only}
-
- Chung BC, Kim KW, Woo UJ, Lee YS, Kim SW, Choi YH, et al.Clinical effects of SJ-002 URI. Korean Journal of Pharmacology 1991;27(2):211-4.
Cohen 2017 {published data only}
-
- Cohen HA, Hoshen M, Gur S, Bahir A, Laks Y, Blau H.Efficacy and tolerability of a polysaccharide-resin-honey based cough syrup as compared to carbocysteine syrup for children with colds: a randomized, single-blinded, multicenter study. World Journal of Pediatrics 2017;13(1):27-33. - PubMed
Connell 1967 {published data only}
-
- Connell JT.Long-acting antihistamine-decongestant evaluation. Archives of Otolaryngology 1967;85(2):218-22. - PubMed
Ghorayeb 2006 {published data only}
-
- Ghorayeb N, Fiss E, De Castro Brandao D.Evaluation of the clinical efficacy and safety of the use of the association between dipiron, caffeine and clorfeniramine maleate compared to the association of paracetamol, chloridrate of fenilefrine and carbinoxamine maleate on the symptomatic treatment of flu and cold. Revista Brasileira de Medicina 2006;63:219-23.
Hosseini 2016 {published data only}
-
- Hosseini F, Mahjoub H, Amanati A, Fazlian MM, Sedighi I.Comparison of zataria multiflora extract syrup and diphenhydramine in the treatment of common cold-induced cough in children: a double-blind, randomized, clinical trial. Archives of Pediatric Infectious Diseases 2016;4(3):e35495.
Kaminszczik 1983 {published data only}
-
- Kaminszczik I, Barbon L.Relieving symptoms of upper respiratory allergies and the common cold: azatadine maleate/pseudoephedrine sulfate syrup versus placebo. Journal of International Medical Research 1983;11(2):101-7. - PubMed
Krishnaprasad 2012 {published data only}
-
- Krishnaprasad K, Manshani P, Karankumar J.Health outcome and safety assessment of a fixed dose combination of amantadine, paracetamol, chlorpheniramine maleate, and phenylephrine introduction in India: a prescription event monitoring study. Perspectives in Clinical Research 2012;3:62-5. - PMC - PubMed
Kuspert 1965 {published data only}
-
- Kuspert A.On the vaso-active therapy of the nasal mucosa with a new adrianol-imidazol combination [Über die vasoaktive Therapie der Nasenschleimhaut mit einer neuartigen Adrianol-Imidazolin-Kombination]. Medizinische Welt 1965;45:2563-4. - PubMed
Lea 1984 {published data only}
-
- Lea P.A double-blind controlled evaluation of the nasal decongestant effect of Day Nurse in the common cold. Journal of International Medical Research 1984;12(2):124-7. - PubMed
Lu 1993 {published data only}
-
- Lu C, Zhiqiang H, Qinming H.Studies on simultaneous determination of paracetamol, caffeine and chlorpheniramine maleate in multicomponent cold-curing medicine by GCS. Chinese Journal of Pharmaceutical Analysis 1993;13:15-8.
Lu 2010 {published data only}
-
- Lu Q, for the Clinical Research Coordination Group of Guaifenesin Compound Pseudoephedrine Hydrochloride Oral Solution.A prospective multicenter randomized controlled clinical study on the efficacy and safety of guaifenesin compound pseudoephedrine hydrochloride oral solution. Zhonghua Erke Zazhi 2010;48:204-7. - PubMed
Mariano 2011 {published data only}
-
- Mariano HG, Solomon G, Steward EC, Albrecht HH.Quality of life in patients taking guaifenesin and pseudoephedrine in an extended-release bi-layer tablet as first-line symptomatic therapy for acute upper respiratory tract infections (URI). Journal of Allergy and Clinical Immunology 2011;127(Suppl 2):AB201. [DOI: 10.1016/j.jaci.2010.12.804] - DOI
McLaurin 1966 {published data only}
-
- McLaurin JW, Graves TA, Komet H.Efficacy of Actifed as a decongestant. Laryngoscope 1966;76(9):1612-4. - PubMed
Mora 1993 {published data only}
-
- Mora A, Priego O, Morales F, Melgarojo MA.Experiences with a combination of astemizol and pseudoephedrine in the treatment of children with rhinitis [Experiencia en ninos con astemizol combinado con pseudoefedrina en el tratamiento de la rinitis]. Investigacion Medica International 1993;20:167-71.
NCT01938144 {unpublished data only}
-
- NCT01938144.Evaluation of the efficacy and safety of two ibuprofen combination products for the treatment of the common cold and flu in Latin America. clinicaltrials.gov/ct2/show/nct01938144 (first received 10 September 2013).
NCT02678234 {published data only}
-
- NCT02678234.An efficacy and safety study of Theraflu Day Powder as oral solution for cold and flu. clinicaltrials.gov/ct2/show/nct02678234 (first received 9 February 2016).
NCT02730364 {published data only}
-
- NCT02730364.An efficacy and safety study of Theraflu Night Powder as oral solution for cold and flu. clinicaltrials.gov/ct2/show/nct02730364 (first received 6 April 2016).
Nelson 1970 {published data only}
Pasotti 1966 {published data only}
-
- Pasotti C, Brembilla E, Corvi G.Sequential clinical analysis of a new product with an antirhinitis action. Il Farmaco 1966;21(1):40-8. - PubMed
Paul 2004 {published data only}
-
- Paul IM, Yoder KE, Crowell KR, Shaffer ML, McMillan HS, Carlson LC, et al.Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics 2004;114(1):e85-90. - PubMed
Peter 1972 {published data only}
-
- Peter M.Treatment of common cold with a new drug Co-Tylenol. Schweizerische Rundschau fur Medizin Praxis 1972;61(20):682-3. - PubMed
Picon 2013 {published data only}
-
- Picon PD, Costa MB, da Veiga Picon R, Costa Cabral Fendt L, Leichter Suksteris M, Carmanim Saccilotto I, et al.Symptomatic treatment of the common cold with a fixed-dose combination of paracetamol, chlorphenamine and phenylephrine: a randomized, placebo-controlled trial. BMC Infectious Diseases 2013;13:556. - PMC - PubMed
Randall 1979 {published data only}
-
- Randall JE, Hendley JO.A decongestant-antihistamine mixture in the prevention of otitis media in children with colds. Pediatrics 1979;63(3):483-5. - PubMed
Sakchainanont 1990 {published data only}
-
- Sakchainanont B, Ruangkanchanasetr S, Chantarojanasiri T, Tapasart C, Suwanjutha S.Effectiveness of antihistamines in common cold. Journal of the Medical Association of Thailand 1990;73(2):96-101. - PubMed
Schachtel 2010 {published data only}
-
- Schachtel BP, Voelker M, Sanner KM, Gagney D, Bey M, Schachtel E, et al.Demonstration of the analgesic efficacy and dose-response of acetylsalicylic acid with pseudoephedrine. Journal of Clinical Pharmacology 2010;50:1429-37. - PubMed
Schuetz 2014 {published data only}
-
- Schuetz P.Randomized controlled trial: neither ibuprofen nor steam improves symptom control compared with paracetamol in patients with acute respiratory tract infection in primary care. BMJ Evidence-Based Medicine 2014;19(3):120. - PubMed
Septimus 2017 {published data only}
-
- Septimus EJ, Albrecht HH, Solomon G, Shea T, Guenin EP.Extended-release guaifenesin/pseudoephedrine hydrochloride for symptom relieve in support of a wait-and-see approach for the treatment of acute respiratory tract infections: a randomized, double-blind, placebo-controlled study. Current Therapeutic Research 2017;84:54-61. - PMC - PubMed
Taborelli 1975 {published data only}
-
- Taborelli G, Chierichetti S.New contributions to the symptomatic treatment of non-specific rhinitis and its complications [Nuovo contributo alla terapia sintomatica delle riniti aspecifiche e delle loro complicazioni]. Minerva Otorinolaringologica 1975;25:266-77.
Todd 1984 {published data only}
-
- Todd JK, Todd N, Damato J, Todd WA.Bacteriology and treatment of purulent nasopharyngitis: a double blind, placebo-controlled evaluation. Pediatric Infectious Disease Journal 1984;3(3):226-32. - PubMed
Virtanen 1982 {published data only}
-
- Virtanen H.The effect of an oral combined preparation (antihistamine and decongestant) on Eustachian tube function in the common cold. ORL: Journal for Oto-Rhino-Laryngology and its Related Specialties 1982;44(5):268-76. - PubMed
Yong 1991 {published data only}
-
- Yong SJ, Lee JG.A clinical study of SJ-002. Korean Journal of Pharmacology 1991;27(2):207-10.
References to studies awaiting assessment
NCT02246166 {published data only}
-
- NCT02246166.The effect of paracetamol, pseudoephedrine hydrochloride, dextromethorphan hydrobromide and chlorpheniramine maleate tablets compared to placebo in subjects suffering from the common cold or influenza. clinicaltrials.gov/show/nct02246166 (first received 22 September 2014).
References to ongoing studies
NCT02904304 {published data only}
-
- NCT02904304.Desloratadine, phenylephrine Hcl, ibuprofen compared to placebo in treatment of symptoms associated with common cold/flu. clinicaltrials.gov/show/nct02904304 (first received 16 September 2016).
Additional references
Atkins 2004
Barret 2005
Barret 2007
Deckx 2016
De Sutter 2015
Eccles 2006b
-
- Eccles R.Efficacy and safety of over-the-counter analgesics in the treatment of common cold and flu. Journal of Clinical Pharmacy and Therapeutics 2006;31:309-19. - PubMed
FDA 2005
-
- Food and Drug Administration, Department of Health and Human Services.21 CFR parts 310, 341, and 357. Phenylpropanolamine-containing drug products for over-the-counter human use. Tentative final monographs. Federal Register/proposed rules 2005;70(245):75988-97.
GRADEpro GDT [Computer program]
-
- GRADEpro GDT.Version accessed 13 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gummin 2018
-
- Gummin D, Mowry J, Spyker D, Brooks D, Beuhler M, Rivers L, et al.2018 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 36th Annual Report. Clinical Toxicology 2019;57(12):1220-413. - PubMed
Gwaltney 2002a
-
- Gwaltney JM.Clinical significance and pathogenesis of viral respiratory infections. American Journal of Medicine 2002;112(Suppl 6A):13-8. - PubMed
Gwaltney 2002b
-
- Gwaltney JM.Viral respiratory infection therapy: historical perspective and current trials. American Journal of Medicine 2002;112(Suppl 6A):33-41. - PubMed
Heikkinnen 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Jacobs 2013
Kim 2015
NIAID 2007
-
- NIAID.Common cold. Review. www3.niaid.nih.gov/healthsicence/healthtopics/colds/overview.htm (accessed 1 February 2007).
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sharfstein 2007
-
- Sharfstein JM, North M, Serwint JR.Over the counter but no longer under the radar - pediatric cough and cold medications. New England Journal of Medicine 2007;357(23):2321-3. - PubMed
Smith 1993
-
- Smith MB, Feldman W.Over-the-counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA 1993;269(17):2258-63. - PubMed
Smith 2014
References to other published versions of this review
De Sutter 2004
-
- De Sutter A, Driel M, Kenyon L.Oral antihistamine-decongestant-analgesic combinations for the common cold. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD004976. [DOI: 10.1002/14651858.CD004976] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

