Third-Generation Antiseizure Medications for Adjunctive Treatment of Focal-Onset Seizures in Adults: A Systematic Review and Network Meta-analysis
- PMID: 35061214
- PMCID: PMC8843918
- DOI: 10.1007/s40265-021-01661-4
Third-Generation Antiseizure Medications for Adjunctive Treatment of Focal-Onset Seizures in Adults: A Systematic Review and Network Meta-analysis
Abstract
Background: Brivaracetam (BRV), cenobamate (CNB), eslicarbazepine acetate (ESL), lacosamide (LCM) and perampanel (PER) are antiseizure medications (ASMs) approved for adjunctive treatment of focal-onset seizures. So far, no randomised controlled trial directly compared the efficacy and safety of these drugs.
Objective: We estimated the comparative efficacy and safety of these ASMs for the treatment of focal-onset seizures in adults with epilepsy using a network meta-analysis (NMA).
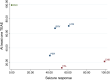
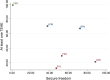
Methods: We systematically searched (June week 4, 2021) MEDLINE (accessed by PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), and the US National Institutes of Health Clinical Trials Registry ( http://www.clinicaltrials.gov ). There were no date limitations or language restrictions. Randomised, double-blinded, controlled, parallel-group, add-on studies that compared oral BRV, CNB, ESL, LCM, and PER versus any comparator over maintenance periods of at least 12 weeks and included adult patients with focal seizures uncontrolled by concomitant ASMs were identified. The efficacy outcomes were the proportions of patients with ≥ 50% and 100% reduction in baseline seizure frequency during the maintenance period. The tolerability outcomes were the proportions of participants who experienced at least one treatment-emergent adverse event (TEAE) and experienced at least one TEAE leading to discontinuation. Effect sizes were estimated by network meta-analyses within a frequentist framework. The hierarchy of competing interventions was established using the surface under the cumulative ranking curve (SUCRA).
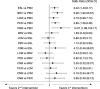
Results: Sixteen trials (BRV: n = 3, CNB: n = 1, ESL: n = 4, LCM: n = 4, PER: n = 4) were included, overall enrolling 4507 patients randomised to add-on active treatments (BRV = 803, CNB = 221, ESL =9 90, LCM = 1104, and PER = 1389) and 2246 to add-on placebo. Cenobamate was associated with a higher rate of ≥ 50% seizure frequency reduction than BRV [odds ratio (OR) 2.02, 95% confidence interval (CI) 1.11-3.66], ESL (OR 1.93, 95% CI 1.07-3.48), LCM (OR 1.86, 95% CI 1.04-3.32), and PER (OR 2.07, 95% CI 1.16-3.70). There was a not statistically significant trend favouring CNB over ESL, LCM and PER for the seizure freedom outcome. Brivaracetam (OR 0.61, 95% CI 0.44-0.86) and LCM (OR 0.60, 95% CI 0.40-0.88) were associated with a lower proportion of participants experiencing TEAEs compared to ESL, and patients treated with PER were associated with a higher risk to experience at least one TEAE (OR 1.42, 95% CI 1.02-1.96) than BRV. According to SUCRA, CNB had the greatest likelihood of being the best option for the ≥ 50% and 100% seizure frequency reduction, and BRV and LCM had the highest probabilities of being the best-tolerated treatments.
Conclusions: Cenobamate ranked best for efficacy, and BRV and LCM were best tolerated over the other comparators. Although NMAs cannot replace direct comparisons, they may support physicians in clinical decision making.
© 2022. The Author(s).
Conflict of interest statement
Simona Lattanzi has received speaker's or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, and UCB Pharma and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, and GW Pharmaceuticals. Eugen Trinka reports personal fees from EVER Pharma, Marinus, Angelini, Arvelle Therapeutics, Argenix, Medtronic, Bial-Portela & Cª, NewBridge, GL Pharma, GlaxoSmithKline, Boehringer Ingelheim, LivaNova, Eisai, Epilog, UCB Pharma, Biogen, Genzyme Sanofi, Takeda, and Actavis; his institution received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Osterreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubiläumsfond der Österreichischen Nationalbank outside the submitted work. Gaetano Zaccara has received speaker’s or consultancy fees from Eisai, Jazz Pharmaceuticals, and UCB Pharma and has served on advisory board for GW Pharmaceuticals. Pasquale Striano received fees and research grants from GW Pharmaceuticals, Zogenyx, Biomarin, and Kolfarma. Emilio Russo has received speaker fees or funding from, and has participated in advisory boards for Angelini, Arvelle Therapeutics, Eisai, Kolfarma, Pfizer, GW Pharmaceuticals, UCB Pharma, and Lundbeck. Francesco Brigo acted as a consultant for Eisai. Cinzia Del Giovane and Mauro Silvestrini have no conflicts of interest directly relevant to the content of this study.
Figures






References
-
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. - PubMed
-
- Cagnetti C, Lattanzi S, Foschi N, Provinciali L, Silvestrini M. Seizure course during pregnancy in catamenial epilepsy. Neurology. 2014;83:339–344. - PubMed
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet. 1995;346:140–144. - PubMed
-
- Lattanzi S, Zaccara G, Giovannelli F, Grillo E, Nardone R, Silvestrini M, Trinka E, Brigo F. Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta-analysis. Acta Neurol Scand. 2019;139:33–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

