Vaccines to prevent COVID-19: A living systematic review with Trial Sequential Analysis and network meta-analysis of randomized clinical trials
- PMID: 35061702
- PMCID: PMC8782520
- DOI: 10.1371/journal.pone.0260733
Vaccines to prevent COVID-19: A living systematic review with Trial Sequential Analysis and network meta-analysis of randomized clinical trials
Abstract
Background: COVID-19 is rapidly spreading causing extensive burdens across the world. Effective vaccines to prevent COVID-19 are urgently needed.
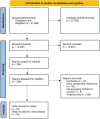
Methods and findings: Our objective was to assess the effectiveness and safety of COVID-19 vaccines through analyses of all currently available randomized clinical trials. We searched the databases CENTRAL, MEDLINE, Embase, and other sources from inception to June 17, 2021 for randomized clinical trials assessing vaccines for COVID-19. At least two independent reviewers screened studies, extracted data, and assessed risks of bias. We conducted meta-analyses, network meta-analyses, and Trial Sequential Analyses (TSA). Our primary outcomes included all-cause mortality, vaccine efficacy, and serious adverse events. We assessed the certainty of evidence with GRADE. We identified 46 trials; 35 trials randomizing 219 864 participants could be included in our analyses. Our meta-analyses showed that mRNA vaccines (efficacy, 95% [95% confidence interval (CI), 92% to 97%]; 71 514 participants; 3 trials; moderate certainty); inactivated vaccines (efficacy, 61% [95% CI, 52% to 68%]; 48 029 participants; 3 trials; moderate certainty); protein subunit vaccines (efficacy, 77% [95% CI, -5% to 95%]; 17 737 participants; 2 trials; low certainty); and viral vector vaccines (efficacy 68% [95% CI, 61% to 74%]; 71 401 participants; 5 trials; low certainty) prevented COVID-19. Viral vector vaccines decreased mortality (risk ratio, 0.25 [95% CI 0.09 to 0.67]; 67 563 participants; 3 trials, low certainty), but comparable data on inactivated, mRNA, and protein subunit vaccines were imprecise. None of the vaccines showed evidence of a difference on serious adverse events, but observational evidence suggested rare serious adverse events. All the vaccines increased the risk of non-serious adverse events.
Conclusions: The evidence suggests that all the included vaccines are effective in preventing COVID-19. The mRNA vaccines seem most effective in preventing COVID-19, but viral vector vaccines seem most effective in reducing mortality. Further trials and longer follow-up are necessary to provide better insight into the safety profile of these vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO. Coronavirus disease (COVID-19) Weekly epidemiological update and weekly operational update [Internet]. 2021 [cited 2021 Aug 3]. https://reliefweb.int/report/world/weekly-operational-update-covid-19-26...
-
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al.. Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS Med. 2020. Sep;17(9):e1003293. doi: 10.1371/journal.pmed.1003293 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials