Activity of Galidesivir in a Hamster Model of SARS-CoV-2
- PMID: 35062212
- PMCID: PMC8780270
- DOI: 10.3390/v14010008
Activity of Galidesivir in a Hamster Model of SARS-CoV-2
Abstract
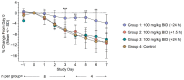
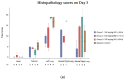
Coronavirus disease 2019 (COVID-19) has claimed the lives of millions of people worldwide since it first emerged. The impact of the COVID-19 pandemic on public health and the global economy has highlighted the medical need for the development of broadly acting interventions against emerging viral threats. Galidesivir is a broad-spectrum antiviral compound with demonstrated in vitro and in vivo efficacy against several RNA viruses of public health concern, including those causing yellow fever, Ebola, Marburg, and Rift Valley fever. In vitro studies have shown that the antiviral activity of galidesivir also extends to coronaviruses. Herein, we describe the efficacy of galidesivir in the Syrian golden hamster model of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Treatment with galidesivir reduced lung pathology in infected animals compared with untreated controls when treatment was initiated 24 h prior to infection. These results add to the evidence of the applicability of galidesivir as a potential medical intervention for a range of acute viral illnesses, including coronaviruses.
Keywords: RNA viruses; SARS-CoV-2; antiviral; coronavirus; nucleoside analog.
Conflict of interest statement
Ray Taylor, MBA, Dennis M. Walling, MD, MMCI, FIDSA, Michael DeSpirito, MS, and Y. S. Babu, Ph.D., are employed by and own stock in BioCryst. James F. Demarest, Ph.D., is a contract consultant for BioCryst. Amanda Mathis, Ph.D., is a former employee of and owns stock in BioCryst. Richard Bowen, DVM, Ph.D., Airn Hartwig, MS, and Helle Bielefeldt-Ohmann, DVM, Ph.D. report no conflicts of interest.
Figures





Similar articles
-
An update on the progress of galidesivir (BCX4430), a broad-spectrum antiviral.Antiviral Res. 2021 Nov;195:105180. doi: 10.1016/j.antiviral.2021.105180. Epub 2021 Sep 20. Antiviral Res. 2021. PMID: 34551346 Free PMC article. Review.
-
An E460D Substitution in the NS5 Protein of Tick-Borne Encephalitis Virus Confers Resistance to the Inhibitor Galidesivir (BCX4430) and Also Attenuates the Virus for Mice.J Virol. 2019 Jul 30;93(16):e00367-19. doi: 10.1128/JVI.00367-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142664 Free PMC article.
-
Pharmacological effect of cepharanthine on SARS-CoV-2-induced disease in a Syrian hamster model.J Infect Chemother. 2025 Jan;31(1):102505. doi: 10.1016/j.jiac.2024.08.020. Epub 2024 Aug 27. J Infect Chemother. 2025. PMID: 39197667
-
Persimmon-derived tannin has antiviral effects and reduces the severity of infection and transmission of SARS-CoV-2 in a Syrian hamster model.Sci Rep. 2021 Dec 8;11(1):23695. doi: 10.1038/s41598-021-03149-3. Sci Rep. 2021. PMID: 34880383 Free PMC article.
-
RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target.J Med Virol. 2021 Jan;93(1):300-310. doi: 10.1002/jmv.26264. Epub 2020 Jul 19. J Med Virol. 2021. PMID: 32633831 Review.
Cited by
-
In silico evaluation of favipiravir-associated potential new drugs against polymerase enzyme of SARS-CoV-2.Heliyon. 2024 Sep 26;10(19):e38479. doi: 10.1016/j.heliyon.2024.e38479. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39398000 Free PMC article.
-
Compellingly high SARS-CoV-2 susceptibility of Golden Syrian hamsters suggests multiple zoonotic infections of pet hamsters during the COVID-19 pandemic.Sci Rep. 2022 Sep 5;12(1):15069. doi: 10.1038/s41598-022-19222-4. Sci Rep. 2022. PMID: 36064749 Free PMC article.
-
Potential RNA-dependent RNA polymerase (RdRp) inhibitors as prospective drug candidates for SARS-CoV-2.Eur J Med Chem. 2023 Apr 5;252:115292. doi: 10.1016/j.ejmech.2023.115292. Epub 2023 Mar 18. Eur J Med Chem. 2023. PMID: 36965227 Free PMC article. Review.
-
Preclinical and Clinical Investigations of Potential Drugs and Vaccines for COVID-19 Therapy: A Comprehensive Review With Recent Update.Clin Pathol. 2024 Jul 26;17:2632010X241263054. doi: 10.1177/2632010X241263054. eCollection 2024 Jan-Dec. Clin Pathol. 2024. PMID: 39070952 Free PMC article. Review.
-
The Virus-Induced Cytopathic Effect.Subcell Biochem. 2023;106:197-210. doi: 10.1007/978-3-031-40086-5_7. Subcell Biochem. 2023. PMID: 38159228
References
-
- COVID-19 Map: Johns Hopkins Coronavirus Resource Center. [(accessed on 8 December 2021)]. Available online: https://coronavirus.jhu.edu/map.html.
-
- Center for Drug Evaluation Research Application Number: 214787Orig1s000: Summary Review. [(accessed on 22 May 2021)]; Available online: Accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000Sumr.pdf.
-
- Emergency Use Authorization for Bamlanivimab 700 mg and Etesevimab 1400 mg IV Administered Together, Center for Drug Evaluation and Research Review. [(accessed on 10 September 2021)]; Available online: https://www.fda.gov/media/146255/download.
-
- Emergency Use Authorization for Casirivimab and Imdevimab, Center for Drug Evaluation Research Review. [(accessed on 10 September 2021)]; Available online: https://www.fda.gov/media/144468/download.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

