Modeling SARS-CoV-2 Infection in Mice Using Lentiviral hACE2 Vectors Infers Two Modes of Immune Responses to SARS-CoV-2 Infection
- PMID: 35062215
- PMCID: PMC8778683
- DOI: 10.3390/v14010011
Modeling SARS-CoV-2 Infection in Mice Using Lentiviral hACE2 Vectors Infers Two Modes of Immune Responses to SARS-CoV-2 Infection
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a severe global pandemic. Mice models are essential to investigate infection pathology, antiviral drugs, and vaccine development. However, wild-type mice lack the human angiotensin-converting enzyme 2 (hACE2) that mediates SARS-CoV-2 entry into human cells and consequently are not susceptible to SARS-CoV-2 infection. hACE2 transgenic mice could provide an efficient COVID-19 model, but are not always readily available, and practically restricted to specific strains. Therefore, there is a dearth of additional mouse models for SARS-CoV-2 infection. We applied lentiviral vectors to generate hACE2 expression in interferon receptor knock-out (IFNAR1-/-) mice. Lenti-hACE2 transduction supported SARS-CoV-2 replication in vivo, simulating mild acute lung disease. Gene expression analysis revealed two modes of immune responses to SARS-CoV-2 infection: one in response to the exposure of mouse lungs to SARS-CoV-2 particles in the absence of productive viral replication, and the second in response to productive SARS-CoV-2 infection. Our results infer that immune response to immunogenic elements on incoming virus or in productively infected cells stimulate diverse immune effectors, even in absence of type I IFN signaling. Our findings should contribute to a better understanding of the immune response triggered by SARS-CoV-2 and to further elucidate COVID-19.
Keywords: COVID-19; SARS-CoV-2; hACE2; immune response; lentivirus; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
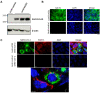
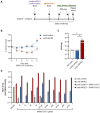

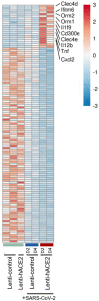
Similar articles
-
SARS-CoV-2 Causes Lung Infection without Severe Disease in Human ACE2 Knock-In Mice.J Virol. 2022 Jan 12;96(1):e0151121. doi: 10.1128/JVI.01511-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668780 Free PMC article.
-
Infectious Clones Produce SARS-CoV-2 That Causes Severe Pulmonary Disease in Infected K18-Human ACE2 Mice.mBio. 2021 Apr 20;12(2):e00819-21. doi: 10.1128/mBio.00819-21. mBio. 2021. PMID: 33879586 Free PMC article.
-
A human-ACE2 knock-in mouse model for SARS-CoV-2 infection recapitulates respiratory disorders but avoids neurological disease associated with the transgenic K18-hACE2 model.mBio. 2025 May 14;16(5):e0072025. doi: 10.1128/mbio.00720-25. Epub 2025 Apr 24. mBio. 2025. PMID: 40272151 Free PMC article.
-
K18- and CAG-hACE2 Transgenic Mouse Models and SARS-CoV-2: Implications for Neurodegeneration Research.Molecules. 2022 Jun 28;27(13):4142. doi: 10.3390/molecules27134142. Molecules. 2022. PMID: 35807384 Free PMC article. Review.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
References
-
- Soldatov V.O., Kubekina M.V., Silaeva Y.Y., Bruter A.V., Deykin A.V. On the way from SARS-CoV-sensitive mice to murine COVID-19 model. Res. Results Pharmacol. 2020;6:1–7. doi: 10.3897/rrpharmacology.6.53633. - DOI
-
- Montagutelli X., Prot M., Levillayer L., Salazar E.B., Jouvion G., Conquet L., Donati F., Albert M., Gambaro F., Behillil S., et al. The B1.351 and P.1 variants extend SARS-CoV-2 host range to mice. bioRxiv. 2021 doi: 10.1101/2021.03.18.436013. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

