Preclinical Assessment of Bacteriophage Therapy against Experimental Acinetobacter baumannii Lung Infection
- PMID: 35062236
- PMCID: PMC8778864
- DOI: 10.3390/v14010033
Preclinical Assessment of Bacteriophage Therapy against Experimental Acinetobacter baumannii Lung Infection
Abstract
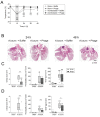
Respiratory infections caused by multidrug-resistant Acinetobacter baumannii are difficult to treat and associated with high mortality among critically ill hospitalized patients. Bacteriophages (phages) eliminate pathogens with high host specificity and efficacy. However, the lack of appropriate preclinical experimental models hampers the progress of clinical development of phages as therapeutic agents. Therefore, we tested the efficacy of a purified lytic phage, vB_AbaM_Acibel004, against multidrug-resistant A. baumannii clinical isolate RUH 2037 infection in immunocompetent mice and a human lung tissue model. Sham- and A. baumannii-infected mice received a single-dose of phage or buffer via intratracheal aerosolization. Group-specific differences in bacterial burden, immune and clinical responses were compared. Phage-treated mice not only recovered faster from infection-associated hypothermia but also had lower pulmonary bacterial burden, lower lung permeability, and cytokine release. Histopathological examination revealed less inflammation with unaffected inflammatory cellular recruitment. No phage-specific adverse events were noted. Additionally, the bactericidal effect of the purified phage on A. baumannii was confirmed after single-dose treatment in an ex vivo human lung infection model. Taken together, our data suggest that the investigated phage has significant potential to treat multidrug-resistant A. baumannii infections and further support the development of appropriate methods for preclinical evaluation of antibacterial efficacy of phages.
Keywords: Acinetobacter baumannii; antibiotic resistance; bacteriophage; pneumonia; preclinical development.
Conflict of interest statement
S.M.W. reports nonfinancial support from Deutsche Gesellschaft für Pneumologie, non-financial support from Mukoviszidose e.V., personal fees and nonfinancial support from Schlütersche Verlagsgesellschaft. G.N. received funding for research from Biotest AG. M.W. received grants and personal fees from Actelion, Bayer Health Care, Biotest AG, Boehringer Ingelheim, NOXXON Pharma, Pantherna, Silence Therapeutics, Vaxxilon, grants from Quark Pharma, Takeda Pharma, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner–Fresenius–Stiftung, CAPNETZ STIFTUNG, International Max Planck Research School, personal fees from Aptarion, AstraZeneca, Berlin Chemie, Chiesi, GlaxoSmithKline, Novartis, Sinoxa and Teva, and has a patent “Means for inhibiting the expression of ANG2 US8829179B2” issued. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. All other authors declare that they have no competing interests.
Figures



References
-
- McCarthy R. Antibiotic resistance: The ‘Other’ Pandemic Lurking behind COVID-19. [(accessed on 1 February 2021)]. Available online: https://bsac.org.uk/antibiotic-resistance-the-other-pandemic-lurking-beh...
-
- Sader H.S., Castanheira M., Arends S.J.R., Goossens H., Flamm R.K. Geographical and temporal variation in the frequency and antimicrobial susceptibility of bacteria isolated from patients hospitalized with bacterial pneumonia: Results from 20 years of the SENTRY Antimicrobial Surveillance Program (1997–2016) J. Antimicrob. Chemother. 2019;74:1595–1606. doi: 10.1093/jac/dkz074. - DOI - PubMed
-
- Ben-Chetrit E., Wiener-Well Y., Lesho E., Kopuit P., Broyer C., Bier L., Assous M.V., Benenson S., Cohen M.J., McGann P.T., et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: Long-term impact for the ICU and hospital. Crit. Care. 2018;22:319. doi: 10.1186/s13054-018-2247-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

