Antidepressant Sertraline Is a Broad-Spectrum Inhibitor of Enteroviruses Targeting Viral Entry through Neutralization of Endolysosomal Acidification
- PMID: 35062313
- PMCID: PMC8780434
- DOI: 10.3390/v14010109
Antidepressant Sertraline Is a Broad-Spectrum Inhibitor of Enteroviruses Targeting Viral Entry through Neutralization of Endolysosomal Acidification
Abstract
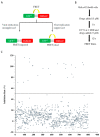
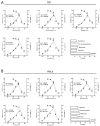
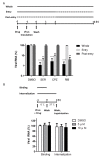
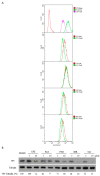
Enterovirus 71 (EV71) is an etiological agent of hand foot and mouth disease and can also cause neurological complications in young children. However, there are no approved drugs as of yet to treat EV71 infections. In this study, we conducted antiviral drug screening by using a Food and Drug Administration (FDA)-approved drug library. We identified five drugs that showed dose-dependent inhibition of viral replication. Sertraline was further characterized because it exhibited the most potent antiviral activity with the highest selectivity index among the five hits. The antiviral activity of sertraline was noted for other EV serotypes. The drug's antiviral effect is not likely associated with its approved indications as an antidepressant and its mode-of-action as a selective serotonin reuptake inhibitor. The time-of-addition assay revealed that sertraline inhibited an EV71 infection at the entry stage. We also showed that sertraline partitioned into acidic compartments, such as endolysosomes, to neutralize the low pH levels. In agreement with the findings, the antiviral effect of sertraline could be greatly relieved by exposing virus-infected cells to extracellular low-pH culture media. Ultimately, we have identified a use for an FDA-approved antidepressant in broad-spectrum EV inhibition by blocking viral entry through the alkalization of the endolysosomal route.
Keywords: antidepressant sertraline; broad-spectrum antiviral; drug repurposing; enterovirus; host-cell targets; viral entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease.J Ethnopharmacol. 2013 May 2;147(1):114-21. doi: 10.1016/j.jep.2013.02.017. Epub 2013 Feb 27. J Ethnopharmacol. 2013. PMID: 23454684 Free PMC article.
-
Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor.Antiviral Res. 2017 Jul;143:122-133. doi: 10.1016/j.antiviral.2017.04.008. Epub 2017 Apr 17. Antiviral Res. 2017. PMID: 28427827
-
Virucidal activity and the antiviral mechanism of acidic polysaccharides against Enterovirus 71 infection in vitro.Microbiol Immunol. 2020 Mar;64(3):189-201. doi: 10.1111/1348-0421.12763. Epub 2020 Jan 14. Microbiol Immunol. 2020. PMID: 31785100
-
Antivirals blocking entry of enteroviruses and therapeutic potential.J Biomed Sci. 2021 Jan 15;28(1):10. doi: 10.1186/s12929-021-00708-8. J Biomed Sci. 2021. PMID: 33451326 Free PMC article. Review.
-
Direct-acting antivirals and host-targeting strategies to combat enterovirus infections.Curr Opin Virol. 2017 Jun;24:1-8. doi: 10.1016/j.coviro.2017.03.009. Epub 2017 Apr 12. Curr Opin Virol. 2017. PMID: 28411509 Free PMC article. Review.
Cited by
-
Fluoxetine and Sertraline Potently Neutralize the Replication of Distinct SARS-CoV-2 Variants.Viruses. 2024 Mar 30;16(4):545. doi: 10.3390/v16040545. Viruses. 2024. PMID: 38675888 Free PMC article.
-
EV-A71 Mechanism of Entry: Receptors/Co-Receptors, Related Pathways and Inhibitors.Viruses. 2023 Mar 18;15(3):785. doi: 10.3390/v15030785. Viruses. 2023. PMID: 36992493 Free PMC article. Review.
-
Activation of store-operated calcium entry and mitochondrial respiration by enterovirus 71 is essential for efficient virus replication.mBio. 2025 Aug 13;16(8):e0371724. doi: 10.1128/mbio.03717-24. Epub 2025 Jul 8. mBio. 2025. PMID: 40626726 Free PMC article.
-
Hand, Foot, and Mouth Disease: A Narrative Review.Recent Adv Inflamm Allergy Drug Discov. 2022;16(2):77-95. doi: 10.2174/1570180820666221024095837. Recent Adv Inflamm Allergy Drug Discov. 2022. PMID: 36284392 Review.
-
Novel virulence determinants in VP1 regulate the assembly of enterovirus-A71.J Virol. 2024 Dec 17;98(12):e0165524. doi: 10.1128/jvi.01655-24. Epub 2024 Nov 13. J Virol. 2024. PMID: 39535185 Free PMC article.
References
-
- Liu S.-L., Pan H., Liu P., Amer S., Chan T.-C., Zhan J., Huo X., Liu Y., Teng Z., Wang L., et al. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev. Med. Virol. 2015;25:115–128. doi: 10.1002/rmv.1827. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

