miR-541-3p Promoted Porcine Reproductive and Respiratory Syndrome Virus 2 (PRRSV-2) Replication by Targeting Interferon Regulatory Factor 7
- PMID: 35062330
- PMCID: PMC8779607
- DOI: 10.3390/v14010126
miR-541-3p Promoted Porcine Reproductive and Respiratory Syndrome Virus 2 (PRRSV-2) Replication by Targeting Interferon Regulatory Factor 7
Abstract
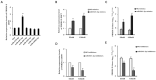
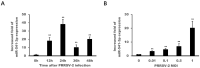
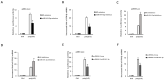
Porcine reproductive and respiratory syndrome (PRRS) is a disease caused by PRRS virus (PRRSV), which seriously harms the pig industry. Revealing the mechanism by which PRRSV inhibits immune response will help prevent and control PRRS. Here, we found that PRRSV-2 may hijack host miR-541-3p to inhibit host innate immune response. Firstly, this work showed that miR-541-3p mimics could facilitate the replication of PRRSV-2 and the results of the quantitative real time polymerase chain reaction (qRT-PCR) showed that PRRSV-2 could up-regulate the expression of miR-541-3p in MARC-145 cells. Since previous studies have shown that type I interferon could effectively inhibit the replication of PRRSV-2, the present work explored whether miR-541-3p regulated the expression of type I interferon and found that miR-541-3p could negatively regulate the transcription of type I interferon by targeting interferon regulatory factor 7 (IRF7). More importantly, PRRSV-2 infection could down-regulate the expression of IRF7 and over-expression of IRF7 could down-regulate the replication of PRRSV-2 in MARC-145 cells. In conclusion, PRRSV-2 infection up-regulated the expression of miR-541-3p to promote its replication in MARC-145 cells, since miR-541-3p can negatively regulate the transcription of type I interferon by targeting IRF7.
Keywords: IRF7; PRRSV; miR-541-3p; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Holtkamp D.J., Kliebenstein J.B., Neumann E.J., Zimmerman J.J., Rotto H.F., Yoder T.K., Wang C., Yeske P.E., Mowrer C.L., Haley C.A. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013;21:72–84.
-
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Adriaenssens E.M., Alfenas-Zerbini P., Davison A.J., Dempsey D.M., Dutilh B.E., Garcia M.L., et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021) Arch. Virol. 2021;166:2633–2648. doi: 10.1007/s00705-021-05156-1. - DOI - PubMed
-
- Chrun T., Maze E.A., Vatzia E., Martini V., Paudyal B., Edmans M.D., McNee A., Manjegowda T., Salguero F.J., Wanasen N., et al. Simultaneous Infection With Porcine Reproductive and Respiratory Syndrome and Influenza Viruses Abrogates Clinical Protection Induced by Live Attenuated Porcine Reproductive and Respiratory Syndrome Vaccination. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.758368. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

