Magnetic Nanoparticles Enhanced Surface Plasmon Resonance Biosensor for Rapid Detection of Salmonella Typhimurium in Romaine Lettuce
- PMID: 35062436
- PMCID: PMC8781532
- DOI: 10.3390/s22020475
Magnetic Nanoparticles Enhanced Surface Plasmon Resonance Biosensor for Rapid Detection of Salmonella Typhimurium in Romaine Lettuce
Abstract
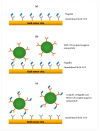
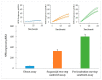
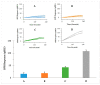
Salmonella is one of the major foodborne pathogens responsible for many cases of illnesses, hospitalizations and deaths worldwide. Although different methods are available to timely detect Salmonella in foods, surface plasmon resonance (SPR) has the benefit of real-time detection with a high sensitivity and specificity. The purpose of this study was to develop an SPR method in conjunction with magnetic nanoparticles (MNPs) for the rapid detection of Salmonella Typhimurium. The assay utilizes a pair of well-characterized, flagellin-specific monoclonal antibodies; one is immobilized on the sensor surface and the other is coupled to the MNPs. Samples of romaine lettuce contaminated with Salmonella Typhimurium were washed with deionized water, and bacterial cells were captured on a filter membrane by vacuum filtration. SPR assays were compared in three different formats-direct assay, sequential two-step sandwich assay, and preincubation one-step sandwich assay. The interaction of flagellin and MNPs with the antibody-immobilized sensor surface were analyzed. SPR signals from a sequential two-step sandwich assay and preincubation one-step sandwich assay were 7.5 times and 14.0 times higher than the direct assay. The detection limits of the assay were 4.7 log cfu/mL in the buffer and 5.2 log cfu/g in romaine lettuce samples.
Keywords: Salmonella Typhimurium; biosensor; flagellin; magnetic nanoparticle; monoclonal antibody; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Andrew W.H., Wang H., Jacobson A., Hammack T. Bacteriological Analytical Manual (BAM): Salmonella. [(accessed on 19 August 2019)]; Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analyti....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

