A Narrative Review of COVID-19 Vaccines
- PMID: 35062723
- PMCID: PMC8779282
- DOI: 10.3390/vaccines10010062
A Narrative Review of COVID-19 Vaccines
Abstract
The COVID-19 pandemic has shaken the world since early 2020 and its health, social, economic, and societal negative impacts at the global scale have been catastrophic. Since the early days of the pandemic, development of safe and effective vaccines was judged to be the best possible tool to minimize the effects of this pandemic. Drastic public health measures were put into place to stop the spread of the virus, with the hope that vaccines would be available soon. Thanks to the extraordinary commitments of many organizations and individuals from around the globe and the collaborative effort of many international scientists, vaccines against COVID-19 received regulatory approval for emergency human use in many jurisdictions in less than a year after the identification of the viral sequence. Several of these vaccines have been in use for some time; however, the pandemic is still ongoing and likely to persist for the foreseeable future. This is due to many reasons including reduced compliance with public health restrictions, limited vaccine manufacturing/distribution capacity, high rates of vaccine hesitancy, and the emergence of new variants with the capacity to spread more easily and to evade current vaccines. Here we discuss the discovery and availability of COVID-19 vaccines and evolving issues around mass vaccination programs.
Keywords: COVID-19; COVID-19 vaccines; SARS-CoV-2; vaccination; vaccine hesitancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


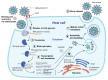
References
-
- World Health Organization WHO Virtual Press Conference on COVID-19. [(accessed on 11 March 2020)]. Available online: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-aud....
-
- World Health Organization Novel Coronavirus 2019. [(accessed on 29 November 2021)]. Available online: https://covid19.who.int/
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

