Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection
- PMID: 35062724
- PMCID: PMC8781423
- DOI: 10.3390/vaccines10010064
Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection
Abstract
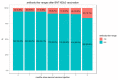
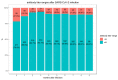
Immune protection following either vaccination or infection with SARS-CoV-2 is thought to decrease over time. We designed a retrospective study, conducted at Leumit Health Services in Israel, to determine the kinetics of SARS-CoV-2 IgG antibodies following administration of two doses of BNT162b2 vaccine, or SARS-CoV-2 infection in unvaccinated individuals. Antibody titers were measured between 31 January 2021, and 31 July 2021 in two mutually exclusive groups: (i) vaccinated individuals who received two doses of BNT162b2 vaccine and had no history of previous infection with COVID-19 and (ii) SARS-CoV-2 convalescents who had not received the vaccine. A total of 2653 individuals fully vaccinated by two doses of vaccine during the study period and 4361 convalescent patients were included. Higher SARS-CoV-2 IgG antibody titers were observed in vaccinated individuals (median 1581 AU/mL IQR [533.8-5644.6]) after the second vaccination than in convalescent individuals (median 355.3 AU/mL IQR [141.2-998.7]; p < 0.001). In vaccinated subjects, antibody titers decreased by up to 38% each subsequent month while in convalescents they decreased by less than 5% per month. Six months after BNT162b2 vaccination 16.1% subjects had antibody levels below the seropositivity threshold of <50 AU/mL, while only 10.8% of convalescent patients were below <50 AU/mL threshold after 9 months from SARS-CoV-2 infection. This study demonstrates individuals who received the Pfizer-BioNTech mRNA vaccine have different kinetics of antibody levels compared to patients who had been infected with the SARS-CoV-2 virus, with higher initial levels but a much faster exponential decrease in the first group.
Keywords: BNT162b2 mRNA vaccine; SARS-CoV-2 infection; antibody titer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, the analyses and interpretation of the data, in the writing of the manuscript, or in the decision to publish the results. A.I., Y.S., I.G., E.M. (Eugene Merzon), A.G.-C., S.V. and E.M. (Eli Magen) are employees of Leumit Health Services. All authors declare that they have no other relationships or activities that could appear to have influenced the submitted work.
Figures


Update of
-
Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection.medRxiv [Preprint]. 2021 Aug 21:2021.08.19.21262111. doi: 10.1101/2021.08.19.21262111. medRxiv. 2021. Update in: Vaccines (Basel). 2021 Dec 31;10(1):64. doi: 10.3390/vaccines10010064. PMID: 34462761 Free PMC article. Updated. Preprint.
References
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

