Anti-SARS-CoV-2 IgG against the S Protein: A Comparison of BNT162b2, mRNA-1273, ChAdOx1 nCoV-2019 and Ad26.COV2.S Vaccines
- PMID: 35062760
- PMCID: PMC8778136
- DOI: 10.3390/vaccines10010099
Anti-SARS-CoV-2 IgG against the S Protein: A Comparison of BNT162b2, mRNA-1273, ChAdOx1 nCoV-2019 and Ad26.COV2.S Vaccines
Abstract
Background: COVID-19 vaccines induce a differentiated humoral and cellular response, and one of the comparable parameters of the vaccine response is the determination of IgG antibodies.
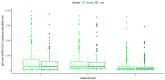
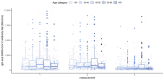
Materials and methods: Concentrations of IgG anti-SARS-CoV-2 antibodies were analyzed at three time points (at the beginning of May, at the end of June and at the end of September). Serum samples were obtained from 954 employees of the Nicolaus Copernicus University in Toruń (a total of three samples each were obtained from 511 vaccinated participants). IgG antibody concentrations were determined by enzyme immunoassay. The statistical analysis included comparisons between vaccines, between convalescents and COVID-19 non-patients, between individual measurements and included the gender, age and blood groups of participants.
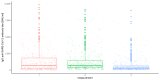
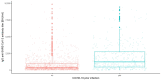
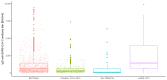
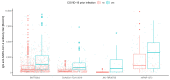
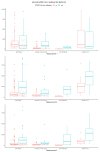
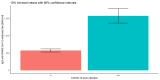
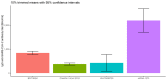
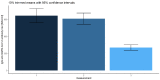
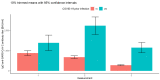
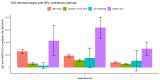
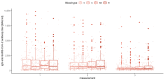
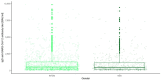
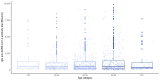
Results: There were significant differences in antibody levels between mRNA and vector vaccines. People vaccinated with mRNA-1273 achieved the highest levels of antibodies, regardless of the time since full vaccination. People vaccinated with ChAdOx1 nCoV-2019 produced several times lower antibody levels compared to the mRNA vaccines, while the antibody levels were more stable. In the case of each of the vaccines, the factor having the strongest impact on the level and stability of the IgG antibody titers was previous SARS-CoV-2 infection. There were no significant correlations with age, gender and blood type.
Summary: mRNA vaccines induce a stronger humoral response of the immune system with the fastest loss of antibodies over time.
Keywords: COVID-19; Janssen/Johnson & Johnson; Moderna; Oxford/AstraZeneca; Pfizer/BioNTech; SARS-CoV-2; antibodies; vaccines.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures






References
-
- Zollner A., Watschinger C., Rossler A., Farcet M.R., Penner A., Bohm V., Kiechl S.J., Stampfel G., Hintenberger R., Tilg H., et al. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine. 2021;70:103539. doi: 10.1016/j.ebiom.2021.103539. - DOI - PMC - PubMed
-
- COVID-19 Vaccination Report. [(accessed on 11 December 2021)]; Available online: https://www.gov.pl/web/szczepimysie/raport-szczepien-przeciwko-covid-19.
LinkOut - more resources
Full Text Sources
Miscellaneous

