Early Activation of the Innate Immunity and Specific Cellular Immune Pathways after Vaccination with a Live Intranasal Viral Vaccine and Challenge with Bovine Parainfluenza Type 3 Virus
- PMID: 35062765
- PMCID: PMC8777984
- DOI: 10.3390/vaccines10010104
Early Activation of the Innate Immunity and Specific Cellular Immune Pathways after Vaccination with a Live Intranasal Viral Vaccine and Challenge with Bovine Parainfluenza Type 3 Virus
Abstract
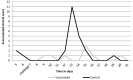
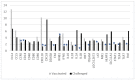
Bovine parainfluenza type 3 (BPIV3) and bovine respiratory syncytial virus (BRSV) may cause bovine respiratory disease (BRD) in very young calves, and therefore vaccination should induce protection at the youngest age and as quickly as possible. This can be achieved by intranasal vaccination with a vaccine containing live attenuated BRSV and BPIV3 virus strains. The objective of this study was to measure gene expression levels by means of RT-qPCR of proteins involved in the innate and adaptive immune response in the nasopharyngeal mucosae after administration of the above-mentioned vaccine and after challenge with BPIV3. Gene expression profiles were different between (i) vaccinated, (ii) nonvaccinated-challenged, and (iii) vaccinated-challenged animals. In nonvaccinated-challenged animals, expression of genes involved in development of disease symptoms and pathology were increased, however, this was not the case after vaccination. Moreover, gene expression patterns of vaccinated animals reflected induction of the antiviral and innate immune pathways as well as an initial Th1 (cytotoxic) cellular response. After challenge with BPIV3, the vaccinated animals were protected against nasal shedding of the challenge virus and clinical symptoms, and in parallel the expression levels of the investigated genes had returned to values that were found before vaccination. In conclusion, in comparison to the virulent wild-type field isolates, the two virus strains in the vaccine have lost their capacity to evade the immune response, resulting in the induction of an antiviral state followed by a very early activation of innate immune and antiviral responses as well as induction of specific cellular immune pathways, resulting in protection. The exact changes in the genomes of these vaccine strains leading to attenuation have not been identified. These data represent the real-life situation and can serve as a basis for further detailed research. This is the first report describing the effects on immune gene expression profiles in the nasal mucosae induced by intranasal vaccination with a bivalent, live BRSV-BPI3V vaccine formulation in comparison to wild-type infection with a virulent BPI3V strain.
Keywords: gene expression; innate immunity; intranasal vaccination; specific immunity.
Conflict of interest statement
All authors are employees of MSD Animal Health, the company that markets the vaccine that has been used in the study reported herein. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Bareille N., Seegers H., Denis G., Quillet J.M., Assie S. Impact of respiratory disorders in young bulls during their fattening period on performance and profitability. Renc. Rech. Rumin. 2008;15:77–80.
LinkOut - more resources
Full Text Sources

