Immunogenicity of a Two-Dose Human Papillomavirus Vaccine Schedule in HIV-Infected Adolescents with Immune Reconstitution
- PMID: 35062779
- PMCID: PMC8779595
- DOI: 10.3390/vaccines10010118
Immunogenicity of a Two-Dose Human Papillomavirus Vaccine Schedule in HIV-Infected Adolescents with Immune Reconstitution
Abstract
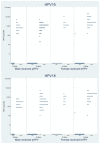
HIV-infected patients are at increased risk of human papillomavirus (HPV) acquisition and HPV-associated diseases. This study set out to determine whether a two-dose (2D) HPV vaccination schedule was sufficient in HIV-infected adolescents with immune reconstitution (IR) following antiretroviral treatment. Participants aged 9-15 years who had CD4 cell counts > 500 cells/mm3 and HIV-1 RNA < 40 copies/mL for at least one year were assigned to the 2D schedule, while older participants or those without IR received a three-dose (3D) schedule. Antibodies to HPV-16 and -18 were measured using a pseudovirion-based neutralization assay. A total of 96 subjects were enrolled; 31.3% and 68.7% received the 2D and 3D schedule, respectively. Of these, 66.7% and 57.6% of the 2D and 3D participants, respectively, were male. The seroconversion rates for HPV-16 and HPV-18 were 100% in all cases, except for HPV-18 in males who received the 3D schedule (97.4%). In males, the anti-HPV-16 geometric mean titers (GMTs) were 6859.3 (95% confidence interval, 4394.3-10,707.1) and 7011.1 (4648.8-10,573.9) in the 2D and 3D groups (p = 0.946), respectively, and the anti-HPV-18 GMTs were 2039.3 (1432.2-2903.8) and 2859.8 (1810.0-4518.4) in the 2D and 3D (p = 0.313) groups, respectively. In females, the anti-HPV-16 GMTs were 15,758.7 (8868.0-28,003.4) and 26,241.6 (16,972.7-40,572.3) in the 2D and 3D groups (p = 0.197), respectively, and the anti-HPV-18 GMTs were 5971.4 (3026.8-11,780.6) and 9993.1 (5950.8-16,781.1) in the 2D and 3D groups (p = 0.271), respectively. In summary, a 2D schedule is as immunogenic in young adolescents with IR as a 3D schedule in older subjects and those without IR.
Keywords: HIV adolescents; HPV in HIV; HPV vaccine; Thai adolescents; two-dose schedule.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Sohn A.H., Kerr S., Hansudewechakul R., Gatechompol S., Chokephaibulkit K., Dang H.L.D., Tran D.N.H., Achalapong J., Teeratakulpisarn N., Chalermchockcharoenkit A., et al. Risk Factors for Human Papillomavirus Infection and Abnormal Cervical Cytology Among Perinatally Human Immunodeficiency Virus-Infected and Uninfected Asian Youth. Clin. Infect. Dis. 2018;67:606–613. doi: 10.1093/cid/ciy144. - DOI - PMC - PubMed
-
- Phanuphak N., Teeraananchai S., Hansudewechakul R., Gatechompol S., Chokephaibulkit K., Dang H.L.D., Tran D.N.H., Achalapong J., Teeratakulpisarn N., Sohn A.H. Incidence and Persistence of High-risk Anogenital Human Papillomavirus Infection Among Female Youth With and Without Perinatally Acquired Human Immunodefiency Virus Infection: A 3-year Observational Cohort Study. Clin. Infect. Dis. 2020;71:e270–e280. doi: 10.1093/cid/ciz1143. - DOI - PMC - PubMed
-
- Gatechompol S., Teeratakulpisarn N., Wittawatmongkol O., Teeraananchai S., Kerr S.J., Chalermchockcharoenkit A., Thamkhantho M., Singtoroj T., Phanuphak N., Sohn A.H., et al. Incidence, persistence, and factors associated with HPV infection among male adolescents with and without perinatally acquired HIV infection. JAIDS J. Acquir. Immune Defic. Syndr. 2020;85:553–560. doi: 10.1097/QAI.0000000000002499. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

