Immunoprofiling Identifies Functional B and T Cell Subsets Induced by an Attenuated Whole Parasite Malaria Vaccine as Correlates of Sterile Immunity
- PMID: 35062785
- PMCID: PMC8780163
- DOI: 10.3390/vaccines10010124
Immunoprofiling Identifies Functional B and T Cell Subsets Induced by an Attenuated Whole Parasite Malaria Vaccine as Correlates of Sterile Immunity
Abstract
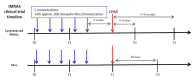
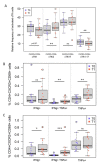
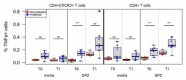
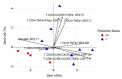
Immune correlates of protection remain elusive for most vaccines. An identified immune correlate would accelerate the down-selection of vaccine formulations by reducing the need for human pathogen challenge studies that are currently required to determine vaccine efficacy. Immunization via mosquito-delivered, radiation-attenuated P. falciparum sporozoites (IMRAS) is a well-established model for efficacious malaria vaccines, inducing greater than 90% sterile immunity. The current immunoprofiling study utilized samples from a clinical trial in which vaccine dosing was adjusted to achieve only 50% protection, thus enabling a comparison between protective and non-protective immune signatures. In-depth immunoprofiling was conducted by assessing a wide range of antigen-specific serological and cellular parameters and applying our newly developed computational tools, including machine learning. The computational component of the study pinpointed previously un-identified cellular T cell subsets (namely, TNFα-secreting CD8+CXCR3-CCR6- T cells, IFNγ-secreting CD8+CCR6+ T cells and TNFα/FNγ-secreting CD4+CXCR3-CCR6- T cells) and B cell subsets (i.e., CD19+CD24hiCD38hiCD69+ transitional B cells) as important factors predictive of protection (92% accuracy). Our study emphasizes the need for in-depth immunoprofiling and subsequent data integration with computational tools to identify immune correlates of protection. The described process of computational data analysis is applicable to other disease and vaccine models.
Keywords: correlate of protection; immune signature; machine learning; malaria; whole sporozoites vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization . World Malaria Report. WHO; Geneva, Switzerland: 2019.
-
- The RTS,S Clinical Trials Partnership Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014;11:e1001685. doi: 10.1371/journal.pmed.1001685. - DOI - PMC - PubMed
-
- The RTS,S Clinical Trials Partnership. Agnandji S.T., Lell B., Fernandes J.F., Abossolo B.P., Methogo B.G., Kabwende A.L., Adegnika A., Mordmuller B., Issifou S., et al. A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N. Engl. J. Med. 2012;367:2284–2295. doi: 10.1056/nejmoa1208394. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

