The effectiveness and safety of corticosteroid therapy for IgA nephropathy with crescents: a prospective, randomized, controlled study
- PMID: 35062886
- PMCID: PMC8780312
- DOI: 10.1186/s12882-022-02661-6
The effectiveness and safety of corticosteroid therapy for IgA nephropathy with crescents: a prospective, randomized, controlled study
Abstract
Background: Pozzi protocol (methylprednisolone intravenous infusion in 1st-3rd-5th months and oral steroid for 6 months) has been thought to be the classic therapy for IgA nephropathy (IgAN) patients with proteinuria> 1.0 g/24 h. There is no consensus on the treatments for IgAN with active pathological changes, especially for IgAN patients with crescents proportion < 50%. This study aimed to evaluate the effectiveness and safety of the treatment protocol of methylprednisolone intravenous infusion at the 1st-2nd-3rd months for IgAN patients with crescents.
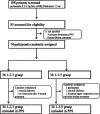
Methods: In this prospective, randomized, controlled, non-blind study, 68 IgAN patients with crescents proportion < 50% were divided into the 1-2-3 group receiving 0.25 g/d methylprednisolone intravenously for 3 consecutive days in the 1st-2nd-3rd months, and oral prednisone 0.5 mg/kg/d on consecutive days for 6 months and the 1-3-5 group with the same intravenous methylprednisolone treatment in the 1st-3rd-5th months, and the same oral prednisone. The primary outcome measure was remission of proteinuria (complete or partial); while the secondary outcome measures were deterioration of renal function (evidenced by a 50% rise from baseline serum creatinine levels, or a 25% decline from baseline eGFR levels).
Results: There was no significant difference in incidence of crescents (median 14.66% vs. 11.45%, p = 0.143) between the 1-2-3 and 1-3-5 groups. From month 1 to month 6, there was a decreasing trend in the levels of urine protein and serum creatinine and an increasing trend in eGFR in both groups. The mean period of remission in the 1-2-3 group seemed shorter. Overall, there were 55 (80.89%) patients meeting remission. The rates of remission in the 1-2-3 group and 1-3-5 group were 85.3 and 76.47%, respectively (P = 0.644). The 1-2-3 group had lower creatinine and higher eGFR than the 1-3-5 group, but the difference was not significant. The complication rate was 11.11 and 15.79% in the two groups, respectively. There was no significant difference in the complications between groups.
Conclusions: Both the 1st-3rd-5th and 1st-2nd-3rd protocols can effectively alleviate proteinuria and protect renal function in IgAN patients with crescents but the 1st-2nd-3rd protocol seemed to have better effectiveness.
Trial registration: ClinicalTrials.gov, Identifier: NCT02160132 , Registered June 10, 2014.
Keywords: Corticosteroid therapy; Crescents; IgA nephropathy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Coppo R, D’Amico G. Factors predicting progression of IgA nephropathies. Journal of Nephrology. 2005;18:503–512. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous