LRG1: an emerging player in disease pathogenesis
- PMID: 35062948
- PMCID: PMC8781713
- DOI: 10.1186/s12929-022-00790-6
LRG1: an emerging player in disease pathogenesis
Abstract
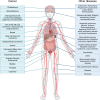
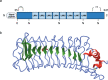
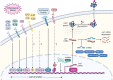
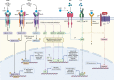
The secreted glycoprotein leucine-rich α-2 glycoprotein 1 (LRG1) was first described as a key player in pathogenic ocular neovascularization almost a decade ago. Since then, an increasing number of publications have reported the involvement of LRG1 in multiple human conditions including cancer, diabetes, cardiovascular disease, neurological disease, and inflammatory disorders. The purpose of this review is to provide, for the first time, a comprehensive overview of the LRG1 literature considering its role in health and disease. Although LRG1 is constitutively expressed by hepatocytes and neutrophils, Lrg1-/- mice show no overt phenotypic abnormality suggesting that LRG1 is essentially redundant in development and homeostasis. However, emerging data are challenging this view by suggesting a novel role for LRG1 in innate immunity and preservation of tissue integrity. While our understanding of beneficial LRG1 functions in physiology remains limited, a consistent body of evidence shows that, in response to various inflammatory stimuli, LRG1 expression is induced and directly contributes to disease pathogenesis. Its potential role as a biomarker for the diagnosis, prognosis and monitoring of multiple conditions is widely discussed while dissecting the mechanisms underlying LRG1 pathogenic functions. Emphasis is given to the role that LRG1 plays as a vasculopathic factor where it disrupts the cellular interactions normally required for the formation and maintenance of mature vessels, thereby indirectly contributing to the establishment of a highly hypoxic and immunosuppressive microenvironment. In addition, LRG1 has also been reported to affect other cell types (including epithelial, immune, mesenchymal and cancer cells) mostly by modulating the TGFβ signalling pathway in a context-dependent manner. Crucially, animal studies have shown that LRG1 inhibition, through gene deletion or a function-blocking antibody, is sufficient to attenuate disease progression. In view of this, and taking into consideration its role as an upstream modifier of TGFβ signalling, LRG1 is suggested as a potentially important therapeutic target. While further investigations are needed to fill gaps in our current understanding of LRG1 function, the studies reviewed here confirm LRG1 as a pleiotropic and pathogenic signalling molecule providing a strong rationale for its use in the clinic as a biomarker and therapeutic target.
Keywords: Cancer; Diabetes; Endothelial cell; Fibrosis; Immunity; Inflammation; LRG1; Neovascularization; Neutrophils; Vascular normalization.
© 2022. The Author(s).
Conflict of interest statement
JG and SEM are founders and members of the scientific advisory board of a company spun out by UCL Business to commercialize a LRG1 function-blocking therapeutic antibody developed through the UK Medical Research Council DPFS funding scheme. JG and SEM are shareholders of this company and named inventors on three patents related to LRG1 as a therapeutic target.
Figures


References
-
- Haupt H, Baudner S. Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author's transl) Hoppe Seylers Z Physiol Chem. 1977;358(6):639–646. - PubMed
-
- Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11(6):725–732. - PubMed
-
- Kallenberg D, Tripathi V, Javaid F, Pilotti C, George J, Davis S, et al. A Humanized Antibody against LRG1 that Inhibits Angiogenesis and Reduces Retinal Vascular Leakage. BioRxiv. 2020;55:137.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

