Infant deaths from respiratory syncytial virus in Lusaka, Zambia from the ZPRIME study: a 3-year, systematic, post-mortem surveillance project
- PMID: 35063114
- PMCID: PMC8789563
- DOI: 10.1016/S2214-109X(21)00518-0
Infant deaths from respiratory syncytial virus in Lusaka, Zambia from the ZPRIME study: a 3-year, systematic, post-mortem surveillance project
Abstract
Background: Respiratory syncytial virus (RSV) is the leading cause of acute lower respiratory tract infections and a key driver of childhood mortality. Previous RSV burden of disease estimates used hospital-based surveillance data and modelled, rather than directly measured, community deaths. Given this uncertainty, we conducted a 3-year post-mortem prevalence study among young infants at a busy morgue in Lusaka, Zambia-the Zambia Pertussis RSV Infant Mortality Estimation (ZPRIME) study.
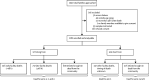
Methods: Infants were eligible for inclusion if they were aged between 4 days and less than 6 months and were enrolled within 48 h of death. Enrolment occurred mainly at the University Teaching Hospital of the University of Zambia Medical School (Lusaka, Zambia), the largest teaching hospital in Zambia. We extracted demographic and clinical data from medical charts and official death certificates, and we conducted verbal autopsies with the guardian or next of kin. RSV was identified using reverse transcriptase quantitative PCR and stratified by age, time of year, and setting (community vs facility deaths). By combining the PCR prevalence data with syndromic presentation, we estimated the proportion of all infant deaths that were due to RSV.
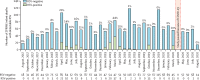
Findings: The ZPRIME study ran from Aug 31, 2017, to Aug 31, 2020, except for from April 1 to May 6, 2020, during which data were not collected due to restrictions on human research at this time (linked to COVID-19). We enrolled 2286 deceased infants, representing 79% of total infant deaths in Lusaka. RSV was detected in 162 (7%) of 2286 deceased infants. RSV was detected in 102 (9%) of 1176 community deaths, compared with 10 (4%) of 236 early facility deaths (<48 h from admission) and 36 (5%) of 737 late facility deaths (≥48 h from admission). RSV deaths were concentrated in infants younger than 3 months (116 [72%] of 162 infants), and were clustered in the first half of each year and in the poorest and most densely populated Lusaka townships. RSV caused at least 2·8% (95% CI 1·0-4·6) of all infant deaths and 4·7% (1·3-8·1) of community deaths.
Interpretation: RSV was a major seasonal cause of overall infant mortality, particularly among infants younger than 3 months of age. Because most RSV deaths occurred in the community and would have been missed through hospital-based surveillance, the global burden of fatal RSV has probably been underestimated.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests CJG and LM report grants from the US National Institutes of Health and from Merck, paid to Boston University. CJG also reports receiving fees for participation on data safety and monitoring boards for Takeda, Moderna, and CureVac, all outside the submitted work. LM reports receiving financial support from the Bill & Melinda Gates Foundation to attend meetings on respiratory syncytial virus. RL reports research awards from Pfizer, paid to her institution, and honoraria for participation in pneumococcal advisory boards and consulting fees from Pfizer, paid to her, outside the submitted work. AL reports employment with Manpower Professional Services, which was contracted by Merck to complete work for the Global Vaccines Public Policy & Partnerships team, and fees from Merck for consultancy services, outside the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Deaths from RSV in young infants-the hidden community burden.Lancet Glob Health. 2022 Feb;10(2):e169-e170. doi: 10.1016/S2214-109X(21)00558-1. Lancet Glob Health. 2022. PMID: 35063106 No abstract available.
References
-
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE, Group WHOCHER WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. - PubMed
-
- Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383:415–425. - PubMed
-
- O'Brien KL, Chandran A, Weatherholtz R, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–1408. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

