Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation
- PMID: 35063115
- PMCID: PMC8789560
- DOI: 10.1016/S2214-109X(21)00517-9
Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation
Abstract
Background: China has the highest prevalence of hepatitis B virus (HBV) infection worldwide. Universal HBV screening might enable China to reach the WHO 2030 target of 90% diagnostics, 80% treatment, and 65% HBV-related death reduction, and eventually elimination of viral hepatitis. We evaluated the cost-effectiveness of implementing universal HBV screening in China and identified optimal screening strategies.
Methods: We used a Markov cohort model, inputting parameters based on data from previous studies and public databases, to assess the cost-effectiveness of four HBV serological screening strategies in China in different screening scenarios. We simulated universal screening scenarios in 15 adult age groups between 18 and 70 years, with different years of screening implementation (2021, 2026, and 2031) and compared to the status quo (ie, no universal screening); in total, we investigated 180 different screening scenarios. We calculated the incremental cost-effectiveness ratio (ICER) between the different screening strategies and the status quo (current screening strategy). We performed probabilistic and one-way deterministic sensitivity analyses to assess the robustness of our findings.
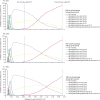
Findings: With a willingness-to-pay level of three times the Chinese gross domestic product (GDP) per capita (US$30 828), all universal screening scenarios in 2021 were cost-effective compared with the status quo. The serum HBsAg/HBsAb/HBeAg/HBeAb/HBcAb (five-test) screening strategy in people aged 18-70 years was the most cost-effective strategy in 2021 (ICER $18 295/quality-adjusted life-years [QALY] gained). This strategy remained the most cost-effective, when the willingness-to-pay threshold was reduced to 2 times GDP per capita. The two-test strategy for people aged 18-70 years became more cost-effective at lower willingness-to-pay levels. The five-test strategy could prevent 3·46 million liver-related deaths in China over the lifetime of the cohort. It remained the most cost-effective strategy when implementation was delayed until 2026 (ICER $20 183/QALY) and 2031 (ICER $23 123/QALY). Screening young people (18-30 years) will no longer be cost-effective in delayed scenarios.
Interpretation: The five-test universal screening strategy in people aged 18-70 years, implemented within the next 10 years, is the optimal HBV screening strategy for China. Other screening strategies could be cost-effective alternatives, if budget is limited in rural areas. Delaying strategy implementation reduces overall cost-effectiveness. Early screening initiation will aid global efforts in achieving viral hepatitis elimination.
Funding: National Natural Science Foundation of China.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests PC is a staff member of WHO; PC alone is responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of WHO. M-FY is an advisory board member or has received research funding from AbbVie, Arbutus Biopharma, Assembly Biosciences, Bristol Myers Squibb, Dicerna Pharmaceuticals, GlaxoSmithKline, Gilead Sciences, Janssen, Merck Sharp and Dohme, ClearB Therapeutics, and Springbank Pharmaceuticals; and has received research funding from Arrowhead Pharmaceuticals, Fujirebio Incorporation, and Sysmex Corporation. W-KS received speaker's fees from AstraZeneca and Mylan, is an advisory board member of CSL Behring, is an advisory board member and received speaker's fees from AbbVie, and is an advisory board member and received speaker's fees and researching funding from Gilead Sciences. FJ received speaker's fees from Gilead Sciences, Merck Sharp and Dohme, and Bristol Myer Squibb, and is an advisory board member of Gilead Sciences and Merck Sharp and Dohme. All remaining authors declare no competing interests.
Figures

Comment in
-
Expanded screening for chronic hepatitis B virus infection in China.Lancet Glob Health. 2022 Feb;10(2):e171-e172. doi: 10.1016/S2214-109X(21)00547-7. Lancet Glob Health. 2022. PMID: 35063107 No abstract available.
-
The optimal screening strategy for chronic hepatitis B virus infection in China.Lancet Glob Health. 2022 Jun;10(6):e792. doi: 10.1016/S2214-109X(22)00169-3. Lancet Glob Health. 2022. PMID: 35561713 No abstract available.
-
The optimal screening strategy for chronic hepatitis B virus infection in China - Authors' reply.Lancet Glob Health. 2022 Jun;10(6):e793. doi: 10.1016/S2214-109X(22)00165-6. Lancet Glob Health. 2022. PMID: 35561714 No abstract available.
References
-
- WHO Hepatitis B key facts. July 27, 2021. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
-
- Chen S, Li J, Wang D, Fung H, Wong LY, Zhao L. The hepatitis B epidemic in China should receive more attention. Lancet. 2018;391 - PubMed
-
- WHO . World Health Organization; Geneva: 2016. Global health sector strategy on viral hepatitis 2016–2021.
-
- Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

