Deep Brain Stimulation for Depression Informed by Intracranial Recordings
- PMID: 35063186
- PMCID: PMC9124238
- DOI: 10.1016/j.biopsych.2021.11.007
Deep Brain Stimulation for Depression Informed by Intracranial Recordings
Abstract
The success of deep brain stimulation (DBS) for treating Parkinson's disease has led to its application to several other disorders, including treatment-resistant depression. Results with DBS for treatment-resistant depression have been heterogeneous, with inconsistencies largely driven by incomplete understanding of the brain networks regulating mood, especially on an individual basis. We report results from the first subject treated with DBS for treatment-resistant depression using an approach that incorporates intracranial recordings to personalize understanding of network behavior and its response to stimulation. These recordings enabled calculation of individually optimized DBS stimulation parameters using a novel inverse solution approach. In the ensuing double-blind, randomized phase incorporating these bespoke parameter sets, DBS led to remission of symptoms and dramatic improvement in quality of life. Results from this initial case demonstrate the feasibility of this personalized platform, which may be used to improve surgical neuromodulation for a vast array of neurologic and psychiatric disorders.
Keywords: Deep brain stimulation; Depression; Epilepsy; Network; Neuromodulation; Stereo-EEG.
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
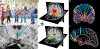
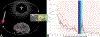
References
-
- Vitek JL, Jain R, Chen L, Troster AI, Schrock LE, House PA, et al. (2020): Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson's disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 19:491–501. - PubMed
-
- Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, et al. (2012): A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 116:315–322. - PubMed
-
- Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhe HG, Notten P, van Laarhoven J, et al. (2016): Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 73:456–464. - PubMed

