Endothelial dysfunction as a complication of anti-cancer therapy
- PMID: 35063569
- PMCID: PMC9294076
- DOI: 10.1016/j.pharmthera.2022.108116
Endothelial dysfunction as a complication of anti-cancer therapy
Abstract
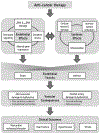
Recent strides in anti-cancer therapeutics have improved longevity and led to a growing population of cancer survivors, who are increasingly likely to die of other causes. Treatment-induced cardiotoxicity is a complication of several therapeutic agents with acute and long-term consequences for cancer patients. Vascular endothelial dysfunction is a precursor and hallmark of ischemic coronary disease and may play a role in anti-cancer therapy-induced cardiotoxicity. This review summarizes clinical evidence for endothelial dysfunction following anti-cancer therapy and extends the discussion to include the impact of therapeutic agents on conduit arteries and the microcirculation. We highlight the role of innate immune system activation and cross-talk between inflammation and oxidative stress as pathogenic mechanisms underlying anti-cancer therapy-induced vascular toxicity. Understanding the impact of anti-cancer agents on the vascular endothelium will inform therapeutic approaches to prevent or reverse treatment-induced cardiotoxicity and may serve as an important tool to predict, monitor, and prevent adverse cardiovascular outcomes in patients undergoing treatment.
Keywords: Cancer-therapy related cardiac dysfunction; Cardio-oncology; Chemotherapy; Endothelium; Inflammation; Microcirculation.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there are no conflicts of interest.
Figures

References
-
- Abou El Hassan MAI, Verheul HMW, Jorna AS, Schalkwijk C, Van Bezu J, Van Der Vijgh WJF, & Bast A (2003). The new cardioprotector Monohydroxyethylrutoside protects against doxorubicin-induced inflammatory effects in vitro. British Journal of Cancer, 89(2), 357–362. doi:10.1038/sj.bjc.6601022 - DOI - PMC - PubMed
-
- Anastasiou M, Oikonomou E, Zagouri F, Siasos G, Antonopoulos AS, Psaltopoulou T, Bamias A, Dimopoulos MA, & Tousoulis D (2017). Flow-mediated dilation of brachial artery as a screening tool for anthracycline-induced cardiotoxicity. Journal of the American College of Cardiology, 70(24), 3072. doi:10.1016/j.jacc.2017.09.1140 - DOI - PubMed
-
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, André F, Tursz T, Kroemer G, & Zitvogel L (2007). The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunological Reviews, 220(1), 47–59. doi:10.1111/j.1600-065X.2007.00573.x - DOI - PubMed
-
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, … Zitvogel L (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Medicine, 13(9), 1050–1059. doi:10.1038/nm1622 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

