Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer
- PMID: 35063964
- PMCID: PMC9365382
- DOI: 10.1158/1078-0432.CCR-21-3213
Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer
Abstract
Purpose: Preoperative chemoradiotherapy (CRT) and surgical resection are the standard treatment for locally advanced rectal cancer (LARC). Combining immune checkpoint inhibitors with radiation suggests a promising approach for enhancing efficacy. We investigated the efficacy of CRT followed by nivolumab and surgery in patients with LARC.
Patients and methods: In phase I, we investigated the feasibility of sequentially combined CRT, 5 cycles of nivolumab, and radical surgery. In phase II, patients with microsatellite stable (MSS) and microsatellite instability-high (MSI-H) LARC were evaluated.
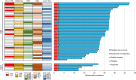
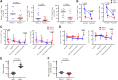
Results: Three patients in phase I received full courses of CRT and nivolumab without dose modification; the schedule was recommended for phase II. A pathologic complete response (pCR) was centrally confirmed in 30% [11/37; 90% confidence interval (CI), 18%-44%] and 60% (3/5) of the MSS and exploratory MSI-H cohorts, respectively. While immune-related severe adverse events were observed in 3 patients, no treatment-related deaths were observed. In 38 patients with MSS who underwent surgery, pCR rates of 75% (6/8) and 17% (5/30; P = 0.004, Fisher exact test) were observed in those with programmed cell death ligand 1 (PD-L1) tumor proportion score ≥1% and <1%, respectively; IHC staining was performed using pre-CRT samples. In 24 patients with MSS, pre-CRT samples were analyzed by flow cytometry; pCR rates of 78% (7/9) and 13% (2/15; P = 0.003, Fisher exact test) were observed for CD8+ T cell/effector regulatory T cell (CD8/eTreg) ratios of ≥2.5 and <2.5, respectively, in tumor-infiltrating lymphocytes.
Conclusions: CRT followed by consolidation nivolumab could increase pCR. PD-L1 expression and an elevated CD8/eTreg ratio were positive predictors in patients with MSS LARC.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
- Clin Cancer Res. 28:1053.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg 1982;69:613–6. - PubMed
-
- Lowry AC, Simmang CL, Boulos P, Farmer KC, Finan PJ, Hyman N, et al. . Consensus statement of definitions for anorectal physiology and rectal cancer: report of the tripartite consensus conference on definitions for anorectal physiology and rectal cancer, Washington, D.C., May 1, 1999. Dis Colon Rectum 2001;44:915–9. - PubMed
-
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

