Influence of methane seepage on isotopic signatures in living deep-sea benthic foraminifera, 79° N
- PMID: 35064198
- PMCID: PMC8782907
- DOI: 10.1038/s41598-022-05175-1
Influence of methane seepage on isotopic signatures in living deep-sea benthic foraminifera, 79° N
Abstract
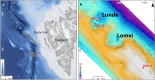
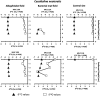
Fossil benthic foraminifera are used to trace past methane release linked to climate change. However, it is still debated whether isotopic signatures of living foraminifera from methane-charged sediments reflect incorporation of methane-derived carbon. A deeper understanding of isotopic signatures of living benthic foraminifera from methane-rich environments will help to improve reconstructions of methane release in the past and better predict the impact of future climate warming on methane seepage. Here, we present isotopic signatures (δ13C and δ18O) of foraminiferal calcite together with biogeochemical data from Arctic seep environments from c. 1200 m water depth, Vestnesa Ridge, 79° N, Fram Strait. Lowest δ13C values were recorded in shells of Melonis barleeanus, - 5.2‰ in live specimens and - 6.5‰ in empty shells, from sediments dominated by aerobic (MOx) and anaerobic oxidation of methane (AOM), respectively. Our data indicate that foraminifera actively incorporate methane-derived carbon when living in sediments with moderate seepage activity, while in sediments with high seepage activity the poisonous sulfidic environment leads to death of the foraminifera and an overgrowth of their empty shells by methane-derived authigenic carbonates. We propose that the incorporation of methane-derived carbon in living foraminifera occurs via feeding on methanotrophic bacteria and/or incorporation of ambient dissolved inorganic carbon.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Stocker, T. F. et al. (eds.). IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1535 (Cambridge University Press, 2013).
-
- Ruppel CD, Kessler JD. The interaction of climate change and methane hydrates. Rev. Geophys. 2017;55:126–168. doi: 10.1002/2016RG000534. - DOI
-
- Biastoch A, et al. Rising Arctic Ocean temperatures cause gas hydrate destabilization and ocean acidification. Geophys. Res. Lett. 2011 doi: 10.1029/2011GL047222. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

