Long-Term Cost Effectiveness of Oral Semaglutide Versus Empagliflozin and Sitagliptin for the Treatment of Type 2 Diabetes in the Swedish Setting
- PMID: 35064550
- PMCID: PMC9043066
- DOI: 10.1007/s41669-021-00317-z
Long-Term Cost Effectiveness of Oral Semaglutide Versus Empagliflozin and Sitagliptin for the Treatment of Type 2 Diabetes in the Swedish Setting
Abstract
Objective: The aim of this study was to assess the cost effectiveness of oral semaglutide versus other oral glucose-lowering drugs for the management of type 2 diabetes (T2D) in Sweden.
Methods: The Swedish Institute for Health Economics Diabetes Cohort Model was used to assess the cost effectiveness of oral semaglutide 14 mg versus empagliflozin 25 mg and oral semaglutide 14 mg versus sitagliptin 100 mg, using data from the head-to-head PIONEER 2 and 3 trials, respectively, in which these treatments were added to metformin (± sulphonylurea). Base-case and scenario analyses were conducted. Robustness was evaluated with deterministic and probabilistic sensitivity analyses.
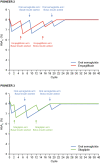
Results: In the base-case analyses, greater initial lowering of glycated haemoglobin levels with oral semaglutide versus empagliflozin and oral semaglutide versus sitagliptin, respectively, resulted in reduced incidences of micro- and macrovascular complications and was associated with lower costs of complications and indirect costs. Treatment costs were higher for oral semaglutide, resulting in higher total lifetime costs than with empagliflozin (Swedish Krona [SEK] 1,245,570 vs. 1,210,172) and sitagliptin (SEK1,405,789 vs. 1,377,381). Oral semaglutide was shown to be cost effective, with an incremental cost-effectiveness ratio (ICER) of SEK239,001 per quality-adjusted life-year (QALY) compared with empagliflozin and SEK120,848 per QALY compared with sitagliptin, from a payer perspective. ICERs were lower at SEK191,721 per QALY compared with empagliflozin and SEK95,234 per QALY compared with sitagliptin from a societal perspective. Results were similar in scenario analyses that incorporated cardiovascular effects, and also in sensitivity analyses.
Conclusions: In a Swedish setting, oral semaglutide was cost effective compared with empagliflozin and sitagliptin for patients with T2D inadequately controlled on oral glucose-lowering drugs.
Trial registration: ClinicalTrials.gov: NCT02863328 (PIONEER 2; registered 11 August 2016) and NCT02607865 (PIONEER 3; registered 18 November 2015).
Plain language summary
For any disease, it is important to consider whether new treatments, which may be more effective but also more expensive, are worth paying for compared with treatments that are already being used. This is called a cost-effectiveness analysis and helps health authorities and other organisations (such as insurance companies) that pay for medications to decide whether or not to pay for the new treatment. Cost effectiveness differs between individual countries because each has its own health system, health costs and approved treatments. Semaglutide is a type of medication called a glucagon-like peptide-1 receptor agonist (or GLP-1RA) that is used by people with type 2 diabetes to help control their blood glucose (sugar). Semaglutide is administered by injection but has recently become the first GLP-1RA to be available in a once-daily oral (tablet) form. We used information from the Swedish Institute for Health Economics and two clinical trials of oral semaglutide that compared it with other oral glucose-lowering drugs—empagliflozin and sitagliptin—to work out whether oral semaglutide was a cost-effective treatment in Sweden. We found that oral semaglutide was more expensive than empagliflozin and sitagliptin over the entire time on treatment but also led to greater lowering of blood sugar. This means that patients had fewer other illnesses linked to diabetes and lower health costs as a result. Balancing the higher upfront cost of the drug versus savings from fewer illnesses linked to diabetes, we found that in Sweden, oral semaglutide was cost effective compared with empagliflozin and sitagliptin.
© 2022. The Author(s).
Conflict of interest statement
Björn Eliasson reports personal fees (expert panels, lectures) from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Mundipharma, Navamedic, NovoNordisk and RLS Global, and grants and personal fees from Sanofi, all outside the submitted work. Åsa Ericsson and Barrie Chubb are employees and shareholders of Novo Nordisk A/S. Adam Fridhammar, Andreas Nilsson and Sofie Persson are employees of the Swedish Institute for Health Economics, which provides consulting services for governmental bodies, academic institutions and commercial life science enterprises (including Novo Nordisk A/S).
Figures
References
-
- Andersson E, Persson S, Hallén N, Ericsson Å, Thielke D, Lindgren P, et al. Costs of diabetes complications: hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia. 2020;63(12):2582–2594. doi: 10.1007/s00125-020-05277-3. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

