Response adaptive intervention allocation in stepped-wedge cluster randomized trials
- PMID: 35064595
- PMCID: PMC7612601
- DOI: 10.1002/sim.9317
Response adaptive intervention allocation in stepped-wedge cluster randomized trials
Abstract
Background: Stepped-wedge cluster randomized trial (SW-CRT) designs are often used when there is a desire to provide an intervention to all enrolled clusters, because of a belief that it will be effective. However, given there should be equipoise at trial commencement, there has been discussion around whether a pre-trial decision to provide the intervention to all clusters is appropriate. In pharmaceutical drug development, a solution to a similar desire to provide more patients with an effective treatment is to use a response adaptive (RA) design.
Methods: We introduce a way in which RA design could be incorporated in an SW-CRT, permitting modification of the intervention allocation during the trial. The proposed framework explicitly permits a balance to be sought between power and patient benefit considerations. A simulation study evaluates the methodology.
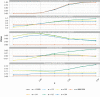
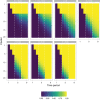
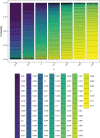
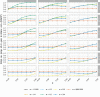
Results: In one scenario, for one particular RA design, the proportion of cluster-periods spent in the intervention condition was observed to increase from 32.2% to 67.9% as the intervention effect was increased. A cost of this was a 6.2% power drop compared to a design that maximized power by fixing the proportion of time in the intervention condition at 45.0%, regardless of the intervention effect.
Conclusions: An RA approach may be most applicable to settings for which the intervention has substantial individual or societal benefit considerations, potentially in combination with notable safety concerns. In such a setting, the proposed methodology may routinely provide the desired adaptability of the roll-out speed, with only a small cost to the study's power.
Keywords: adaptive design; clinical trial; interim analysis; multi-stage; sequential allocation.
© 2022 The Authors. Statistics in Medicine published by John Wiley & Sons Ltd.
Figures


References
-
- Kotz D, Spigt M, Arts I, Crutzen R, Viechtbauer W. Use of the stepped wedge design cannot be recommended: a critical appraisal and comparison with the classic cluster randomized controlled trial design. J Clin Epidemiol. 2012;65:1249‐1252. - PubMed
-
- Mdege N, Man MS, Taylor nee Brown C, Torgerson D. There are some circumstances where the stepped‐wedge cluster randomized trial is preferable to the alternative: no randomized trial at all. response to the commentary by Kotz and colleagues. J Clin Epidemiol. 2012;65:1253‐1254. - PubMed
-
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141‐145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
