Effects of shell-integrated Sudan Black dye on the acoustic activity and ultrasound imaging properties of lipid-shelled nanoscale ultrasound contrast agents
- PMID: 35064656
- PMCID: PMC8781525
- DOI: 10.1117/1.JBO.27.1.016501
Effects of shell-integrated Sudan Black dye on the acoustic activity and ultrasound imaging properties of lipid-shelled nanoscale ultrasound contrast agents
Abstract
Significance: An effective contrast agent for concurrent multimodal photoacoustic (PA) and ultrasound (US) imaging must have both high optical absorption and high echogenicity. Integrating a highly absorbing dye into the lipid shell of gas core nanobubbles (NBs) adds PA contrast to existing US contrast agents but may impact agent ultrasonic response.
Aim: We report on the development and ultrasonic characterization of lipid-shell stabilized C3F8 NBs with integrated Sudan Black (SB) B dye in the shell as dual-modal PA-US contrast agents.
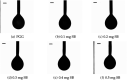
Approach: Perfluoropropane NBs stabilized with a lipid shell including increasing concentrations of SB B dye were formulated by amalgamation (SBNBs). Physical properties of SBNBs were characterized using resonant mass measurement, transmission electron microscopy and pendant drop tensiometry. Concentrated bubble solutions were imaged for 8 min to assess signal decay. Diluted bubble solutions were stimulated by a focused transducer to determine the response of individual NBs to long cycle (30 cycle) US. For assessment of simultaneous multimodal contrast, bulk populations of SBNBs were imaged using a PA and US imaging platform.
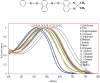
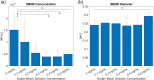
Results: We produced high agent yield (∼1011) with a mean diameter of ∼200 to 300 nm depending on SB loading. A 40% decrease in bubble yield was measured for solutions with 0.3 and 0.4 mg / ml SB. The addition of SB to the shell did not substantially affect NB size despite an increase in surface tension by up to 8 mN / m. The bubble decay rate increased after prolonged exposure (8 min) by dyed bubbles in comparison to their undyed counterparts (2.5-fold). SB in bubble shells increased gas exchange across the shell for long cycle US. PA imaging of these agents showed an increase in power (up to 10 dB) with increasing dye.
Conclusions: We added PA contrast function to NBs. The addition of SB increased gas exchange across the NB shell. This has important implications in their use as multimodal agents.
Keywords: contrast agents; multimodal imaging; nanobubbles; photoacoustic imaging; rectified diffusion; ultrasound imaging.
Figures








References
-
- Szabo T. L., Diagnostic Ultrasound Imaging: Inside Out, Elsevier Academic Press, San Diego, California: (2004).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

