Nephron mass determines the excretion rate of urinary extracellular vesicles
- PMID: 35064766
- PMCID: PMC8783354
- DOI: 10.1002/jev2.12181
Nephron mass determines the excretion rate of urinary extracellular vesicles
Abstract
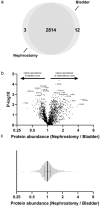
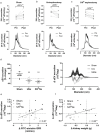
Urinary extracellular vesicles (uEVs) are emerging as non-invasive biomarkers for various kidney diseases, but it is unknown how differences in nephron mass impact uEV excretion. To address this, uEV excretion was measured before and after human kidney donor nephrectomy and rat nephrectomy. In male and female donors, uEVs were quantified in cell-free spot and 24-h urine samples using nanoparticle tracking analysis (NTA), EVQuant, and CD9-time-resolved fluorescence immunoassay. Female donors had significantly lower total kidney volume (TKV) and excreted 49% fewer uEVs than male donors. uEV excretion correlated positively with estimated glomerular filtration rate (eGFR), creatinine clearance, and TKV (R's between 0.6 and 0.7). uEV excretion rate could also be predicted from spot urines after multiplying spot uEV/creatinine by 24-h urine creatinine. Donor nephrectomy reduced eGFR by 36% ± 10%, but the excretion of uEVs by only 16% (CD9+ uEVs -37%, CD9- uEVs no decrease). Donor nephrectomy increased the podocyte marker WT-1 and the proximal tubule markers NHE3, NaPi-IIa, and cubilin in uEVs two- to four-fold when correcting for the nephrectomy. In rats, the changes in GFR and kidney weight correlated with the changes in uEV excretion rate (R = 0.46 and 0.60, P < 0.01). Furthermore, the estimated degree of hypertrophy matched the change in uEV excretion rate (1.4- to 1.5-fold after uninephrectomy and four-fold after 5/6th nephrectomy). Taken together, our data show that uEV excretion depends on nephron mass, and that nephrectomy reduces uEV excretion less than expected based on nephron loss due to compensatory hypertrophy. The major implication of our findings is that a measure for nephron mass or uEV excretion rate should be included when comparing uEV biomarkers between individuals.
Keywords: creatinine; excretion rate; exosomes; kidney; kidney function; nephrectomy; normalization; quantification.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Figures




References
-
- Bertram, J. F. , Douglas‐Denton, R. N. , Diouf, B. , Hughson, M. D. , & Hoy, W. E. (2011). Human nephron number: Implications for health and disease. Pediatric Nephrology, 26, 1529–1533. - PubMed
-
- Blijdorp, C. J. , & Hoorn, E. J. (2019). Urinary extracellular vesicles: The mothership connection. American Journal of Physiology. Renal Physiology, 317, F648‐F649. - PubMed
-
- Blijdorp, C. J. , Tutakhel, O. A. Z. , Hartjes, T. A. , Van Den Bosch, T. P. P. , Van Heugten, M. H. , Rigalli, J. P. , Willemsen, R. , Musterd‐Bhaggoe, U. M. , Barros, E. R. , Carles‐Fontana, R. , Carvajal, C. A. , Arntz, O. J. , Van De Loo, F. A. J. , Jenster, G. , Clahsen‐Van Groningen, M. C. , Cuevas, C. A. , Severs, D. , Fenton, R. A. , Van Royen, M. E. , … Hoorn, E. J. (2021). Comparing approaches to normalize, quantify, and characterize urinary extracellular vesicles. Journal of the American Society of Nephrology, 32, 1210–1226. - PMC - PubMed
-
- Boudville, N. , Ramesh Prasad, G. V. , Knoll, G. , Muirhead, N. , Thiessen‐Philbrook, H. , Yang, R. C. , Rosas‐Arellano, M. P , Housawi, A. , & Garg, A. X. (2006). Meta‐analysis: Risk for hypertension in living kidney donors. Annals of Internal Medicine, 145, 185–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

