Utility and Impact of the Implementation of Same-Day, Self-administered Electronic Patient-Reported Outcomes Assessments in Routine HIV Care in two North American Clinics
- PMID: 35064851
- PMCID: PMC8783196
- DOI: 10.1007/s10461-022-03585-w
Utility and Impact of the Implementation of Same-Day, Self-administered Electronic Patient-Reported Outcomes Assessments in Routine HIV Care in two North American Clinics
Abstract
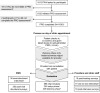
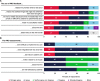
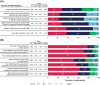
The PROgress study assessed the value and feasibility of implementing web-based patient-reported outcomes assessments (PROs) within routine HIV care at two North American outpatient clinics. People with HIV (PWH) completed PROs on a tablet computer in clinic before their routine care visit. Data collection included PROs from 1632 unique PWH, 596 chart reviews, 200 patient questionnaires, and 16 provider/staff questionnaires. During an initial setup phase involving 200 patients, PRO results were not delivered to providers; for all subsequent patients, providers received PRO results before the consultation. Chart review demonstrated that delivery of PRO results to providers improved patient-provider communication and increased the number of complex health and behavioral issues identified, recorded, and acted on, including suicidal ideation (88% with vs 38% without PRO feedback) and anxiety (54% with vs 24% without PRO feedback). In post-visit questionnaires, PWH (82%) and providers (82%) indicated that the PRO added value to the visit.
Keywords: HIV care; Implementation science; Patient-reported outcomes; Quality of life; Suicidal ideation.
© 2022. The Author(s).
Conflict of interest statement
DS is an employee of ViiV Healthcare and may own stock in GlaxoSmithKline. HC has received grants from ViiV Healthcare. SS has received grants from the Ontario HIV Treatment Network (OHTN). MR has received personal fees from Gilead, ViiV Healthcare, Janssen, and Merck for speaking engagements and/or consultancies. JBe has received personal fees from Merck, ViiV Healthcare, and Gilead for advisory board participation. BJ is an employee of Midway Immunology and Research Center, which was hired by Midway Specialty Care Center to oversee this project. RF, EF, JBa, AM, KG, JM, AK, VH, DK, and WL have nothing to disclose.
Figures


References
-
- Grinsztejn B, De Castro N, Arnold V, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14(6):459–467. doi: 10.1016/S1473-3099(14)70711-X. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

