Parallel evolution of two distinct lymphoid proliferations in clonal haematopoiesis
- PMID: 35064935
- PMCID: PMC9310594
- DOI: 10.1111/his.14619
Parallel evolution of two distinct lymphoid proliferations in clonal haematopoiesis
Abstract
Aims: Angioimmunoblastic T-cell lymphoma (AITL) is genetically characterized by TET2 and DNMT3A mutations occurring in haematopoietic progenitor cells, and late events (e.g. the RHOA-G17V mutation) associated with malignant transformation. As TET2/DNMT3A-mutated progenitor cells can differentiate into multilineage progenies and give rise to both AITL and myeloid neoplasms, they may also have the potential to lead to other metachronous/synchronous neoplasms. We report two cases showing parallel evolution of two distinct potentially neoplastic lymphoid proliferations from a common mutated haematopoietic progenitor cell population.
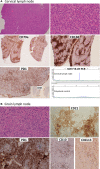
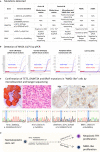
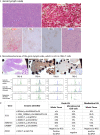
Methods and results: Both cases presented with generalized lymphadenopathy. In case 1 (a 67-year-old female), an initial lymph node (LN) biopsy was dismissed as reactive, but a repeat biopsy showed a nodal marginal zone lymphoma (NMZL)-like proliferation with an increase in the number of T-follicular helper (TFH) cells. Immunohistochemistry, and clonality and mutational analyses by targeted sequencing of both whole tissue sections and microdissected NMZL-like lesions, demonstrated a clonal B-cell proliferation that harboured the BRAF-G469R mutation and shared TET2 and DNMT3A mutations with an underlying RHOA-G17V-mutant TFH proliferation. Review of the original LN biopsy showed histological and immunophenotypic features of AITL. In case 2 (a 66-year-old male), cytotoxic T-cell lymphoma with an increase in the number of Epstein-Barr virus-positive large B cells was diagnosed on initial biopsy. On review together with the relapsed biopsy, we identified an additional occult neoplastic TFH proliferation/smouldering AITL. Both T-cell proliferations shared TET2 and DNMT3A mutations while RHOA-G17V was confined to the smouldering AITL.
Conclusions: In addition to demonstrating diagnostic challenges, these cases expand the potential of clonal haematopoiesis in the development of different lineage neoplastic proliferations.
Keywords: TET2 and DNMT3A mutation; angioimmunoblastic T-cell lymphoma; clonal haematopoiesis; secondary lymphoid neoplasm.
© 2022 The Authors. Histopathology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Dogan A, Gaulard P, Jaffe ES et al. Angioimmunoblastic T‐cell lymphoma and other nodal lymphomas of T follicular helper origin. In Swerdlow S, Campo E, Harris NL et al. eds. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press, 2017; 407–412.
-
- Attygalle AD, Kyriakou C, Dupuis J et al. Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am. J. Surg. Pathol. 2007; 31; 1077–1088. - PubMed
-
- Sakata‐Yanagimoto M, Enami T, Yoshida K et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat. Genet. 2014; 46; 171–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

